AP Chemistry 2020 2021 Anthony Petras Day 14






- Slides: 15

AP Chemistry 2020 -2021 Anthony Petras

Day 14 • Attendance • Recording On • 2. 7 VSEPR & Bond Hybridization Practice • Homework – Isn’t all work “homework”? • Video Links • phet “lab”: https: //phet. colorado. edu/en/simulation/moleculeshapes • Test 10/27/2020

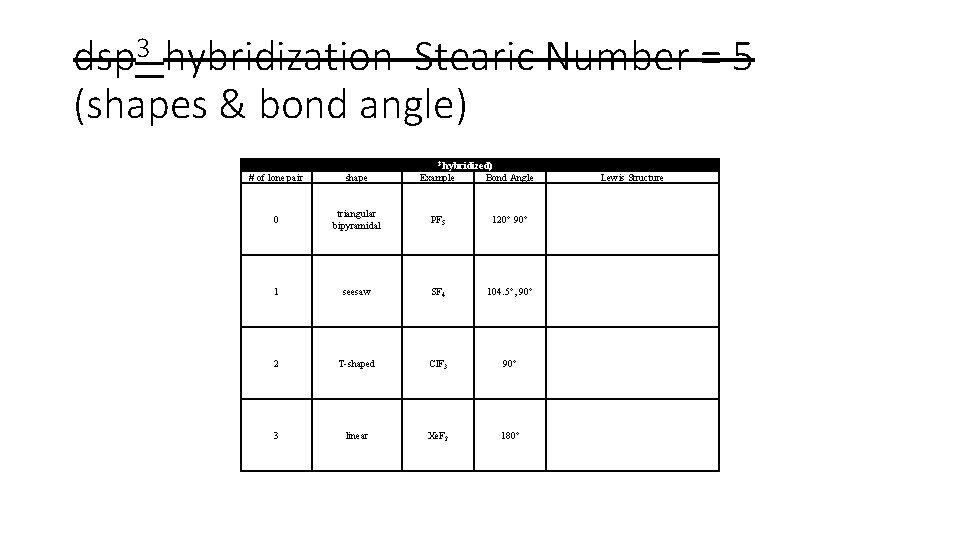
dsp 3 hybridization Stearic Number = 5 (shapes & bond angle) Central Atoms with Five Effective Pairs (dsp 3 hybridized) # of lone pair shape Example Bond Angle 0 triangular bipyramidal PF 5 120° 90° 1 seesaw SF 4 104. 5°, 90° 2 T-shaped Cl. F 3 90° 3 linear Xe. F 2 180° Lewis Structure

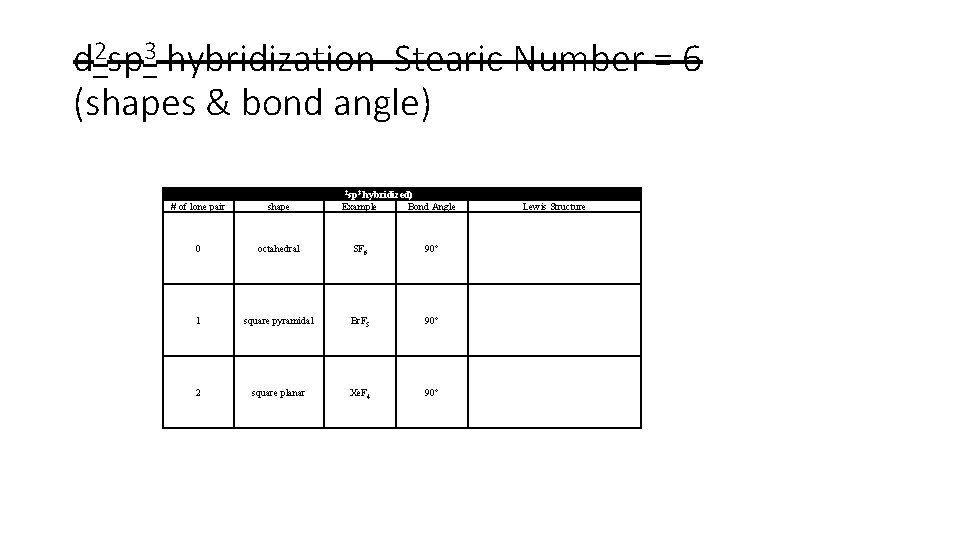
d 2 sp 3 hybridization Stearic Number = 6 (shapes & bond angle) Central Atoms with Six Effective Pairs (d 2 sp 3 hybridized) # of lone pair shape Example Bond Angle 0 octahedral SF 6 90° 1 square pyramidal Br. F 5 90° 2 square planar Xe. F 4 90° Lewis Structure

Molecular Geometry Practice #1 -5 • Use the following answers for questions 1 - 5. Choose the molecular geometry for each of the following molecules. You may use an answer more than once. • (A) T-shaped (B) see-saw (C) square pyramidal (D) trigonal planar (E) trigonal bipyramidal • • • 1. IF 5 2. SF 4 3. PF 5 4. Cl. F 3 5. NO 31 -

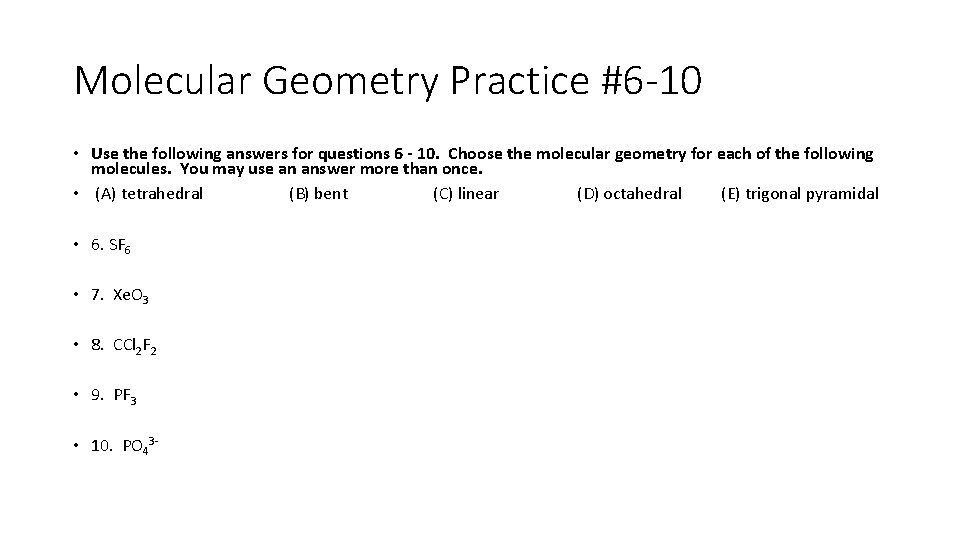
Molecular Geometry Practice #6 -10 • Use the following answers for questions 6 - 10. Choose the molecular geometry for each of the following molecules. You may use an answer more than once. • (A) tetrahedral (B) bent (C) linear (D) octahedral (E) trigonal pyramidal • • • 6. SF 6 7. Xe. O 3 8. CCl 2 F 2 9. PF 3 10. PO 43 -

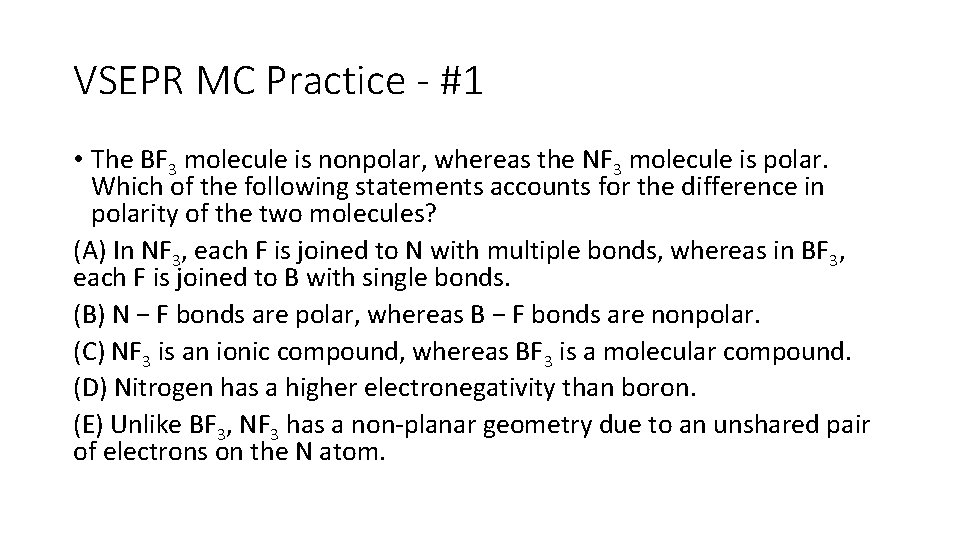
VSEPR MC Practice - #1 • The BF 3 molecule is nonpolar, whereas the NF 3 molecule is polar. Which of the following statements accounts for the difference in polarity of the two molecules? (A) In NF 3, each F is joined to N with multiple bonds, whereas in BF 3, each F is joined to B with single bonds. (B) N − F bonds are polar, whereas B − F bonds are nonpolar. (C) NF 3 is an ionic compound, whereas BF 3 is a molecular compound. (D) Nitrogen has a higher electronegativity than boron. (E) Unlike BF 3, NF 3 has a non-planar geometry due to an unshared pair of electrons on the N atom.

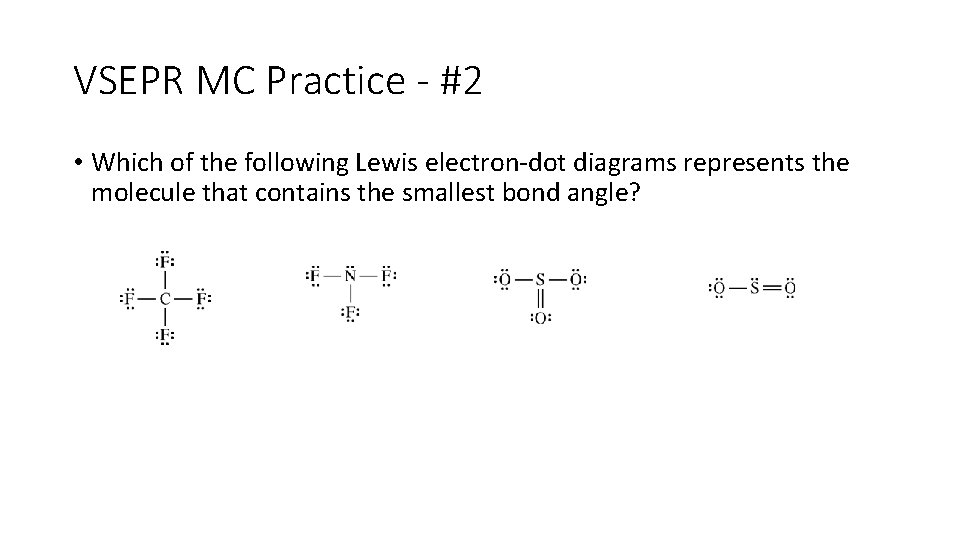
VSEPR MC Practice - #2 • Which of the following Lewis electron-dot diagrams represents the molecule that contains the smallest bond angle?

VSEPR MC Practice - #3 C 2 H 2(g) + Cl 2(g) C 2 H 2 Cl 2(g) • When the reaction above occurs, does the hybridization of the carbon atoms change? (A) Yes; it changes from sp to sp 2 (B) Yes; it changes from sp to sp 3 (C) Yes; it changes from sp 3 to sp 2 (D) Yes; it changes from sp 2 to sp 3 (E) No; it does not change

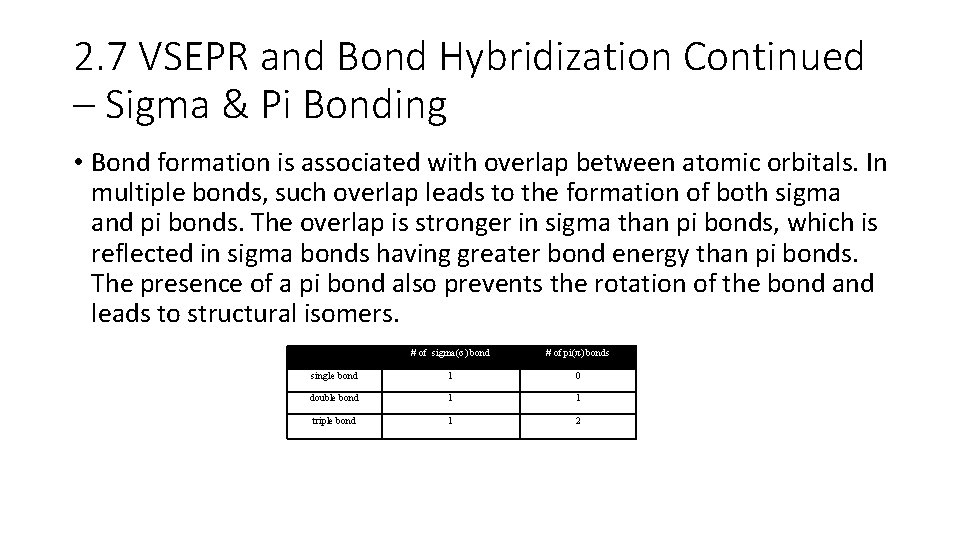
2. 7 VSEPR and Bond Hybridization Continued – Sigma & Pi Bonding • Bond formation is associated with overlap between atomic orbitals. In multiple bonds, such overlap leads to the formation of both sigma and pi bonds. The overlap is stronger in sigma than pi bonds, which is reflected in sigma bonds having greater bond energy than pi bonds. The presence of a pi bond also prevents the rotation of the bond and leads to structural isomers. Type of bond # of sigma(σ) bond # of pi(π) bonds single bond 1 0 double bond 1 1 triple bond 1 2

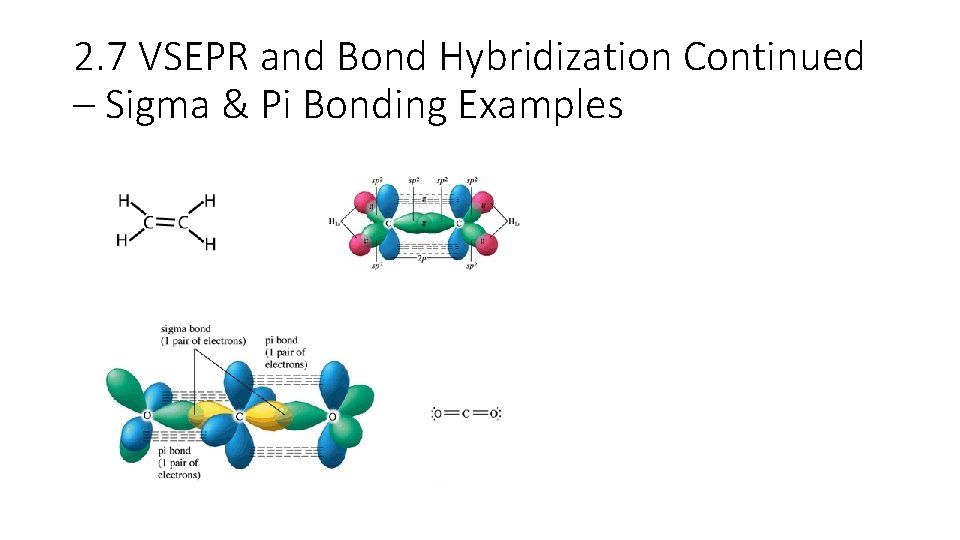
2. 7 VSEPR and Bond Hybridization Continued – Sigma & Pi Bonding Examples

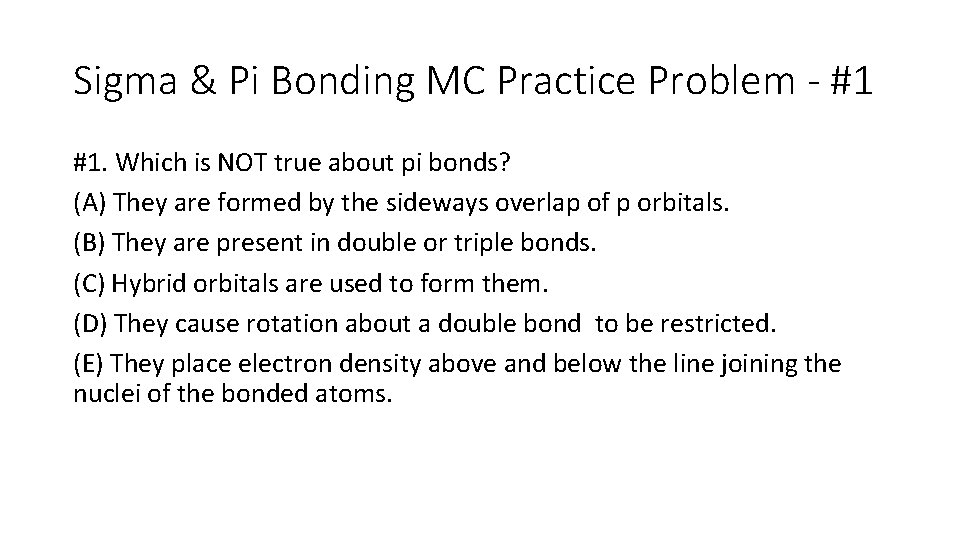
Sigma & Pi Bonding MC Practice Problem - #1 #1. Which is NOT true about pi bonds? (A) They are formed by the sideways overlap of p orbitals. (B) They are present in double or triple bonds. (C) Hybrid orbitals are used to form them. (D) They cause rotation about a double bond to be restricted. (E) They place electron density above and below the line joining the nuclei of the bonded atoms.

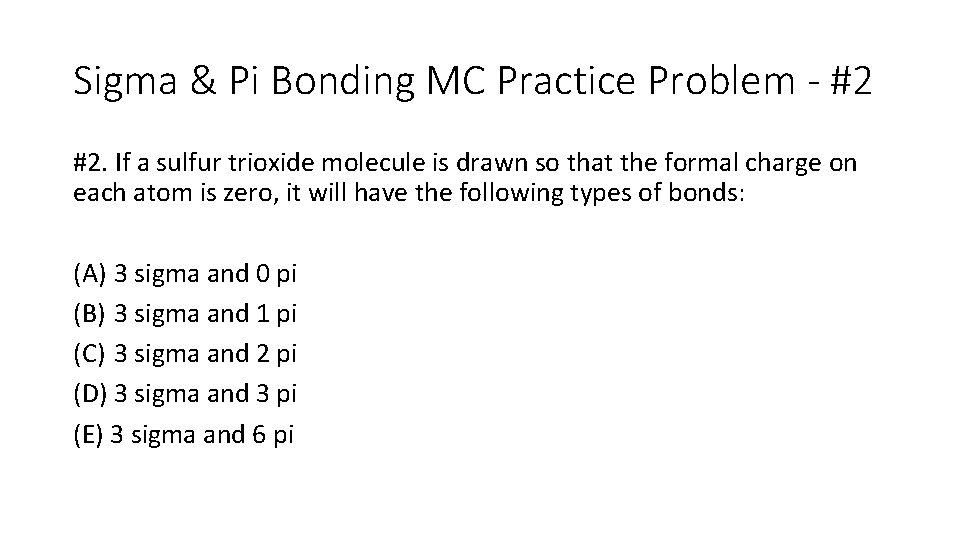
Sigma & Pi Bonding MC Practice Problem - #2 #2. If a sulfur trioxide molecule is drawn so that the formal charge on each atom is zero, it will have the following types of bonds: (A) 3 sigma and 0 pi (B) 3 sigma and 1 pi (C) 3 sigma and 2 pi (D) 3 sigma and 3 pi (E) 3 sigma and 6 pi

Homework – Day 14 • Review this Power. Point • Available Resources: (NOT REQUIRED) • Sartep AP chapter 8 worksheets • Chemistry Honors chapter 5 worksheets • Office Hours Monday 8 -11 am • Test Tuesday 10/27 Chapter 8 Outline Chapter 8 Problem Set Extra Credit 8_01 Answers Extra Credit 8_02 Answers Extra Credit 8_03 Answers Chapter 8 HW #1 Chapter 8 HW #2 Answers Warm Up 8_01 Answers Warm Up 8_02 Answers Chapter 8 Text Book Multiple Choice

Recording Links • • • • Period 1: 14. https: //us. bbcollab. com/recording/cb 233 aaa 50 df 49 a 0 afe 1 fdad 5421072 e 13. https: //us. bbcollab. com/recording/7376 c 83 fd 95640358851 fffaca 15 b 43 a 12. https: //us. bbcollab. com/recording/1 b 005 d 95 b 4 b 54 c 389 e 70 bb 650 e 9 d 01 e 7 11. https: //us. bbcollab. com/recording/4 f 46 e 8 c 951 a 0423 c 80 a 9 a 65178 d 7 f 107 10. https: //us. bbcollab. com/recording/80532 ccee 7 d 24186 a 0 c 5548169688197 9. https: //us. bbcollab. com/recording/f 85 e 338658 e 5456 ea 5 a 5 d 9 b 03 d 004928 8. https: //us. bbcollab. com/recording/3 fac 39 a 2 a 35240 f 6 b 076 d 0 deb 10905 da 7. https: //us. bbcollab. com/recording/53 f 201467 e 3 f 4242 b 98246 f 68 e 87 e 81 a 6. https: //us. bbcollab. com/recording/17 a 57 e 3670094 ab 2 a 4 df 862 b 3 b 64 c 49 c 5. https: //us. bbcollab. com/recording/fad 4 d 7 a 6 ae 754 b 08 a 83 ceb 987 d 46 d 8 a 0 4. https: //us. bbcollab. com/recording/a 197 d 2 ccfafc 453 cb 90173 e 32 d 2 a 85 ec 3. https: //us. bbcollab. com/recording/2 b 83 c 7 af 5450418187 f 24445263 ccdb 3 2. https: //us. bbcollab. com/recording/76 b 616 c 2 b 45343 c 38510 aad 2518 db 114 1. https: //us. bbcollab. com/recording/b 5 ff 705 fea 0 a 435 f 9 b 581 e 27 b 3 df 2 a 30
 Anthony petras
Anthony petras Anthony petras
Anthony petras Day 1 day 2 day 3 day 4
Day 1 day 2 day 3 day 4 Day 1 day 2 day 817
Day 1 day 2 day 817 Petras tehachapi
Petras tehachapi Petras dovydaitis
Petras dovydaitis Miškais ateina ruduo konspektas
Miškais ateina ruduo konspektas Petras cvirka biografija
Petras cvirka biografija Jeremy petras
Jeremy petras Dr petras kisielius
Dr petras kisielius Vivien petras
Vivien petras Liudna pasaka trumpas aprasymas
Liudna pasaka trumpas aprasymas Kas
Kas Teacher goals for 2020-2021
Teacher goals for 2020-2021 Nfhs volleyball uniform rules 2020-2021
Nfhs volleyball uniform rules 2020-2021 Elearning utmspace
Elearning utmspace