Antiepileptic Drug Selection for People with HIVAIDS Report































- Slides: 43

Antiepileptic Drug Selection for People with HIV/AIDS Report of the American Academy of Neurology and the International League Against Epilepsy Gretchen L. Birbeck, MD, MPH, DTMH, FAAN; Jacqueline A. French, MD, FAAN; Emilio Perucca, MD, Ph. D, FRCP(Edin); David M. Simpson, MD; Henry Fraimow, MD; Jomy M. George, Pharm. D, BCPS; Jason F. Okulicz, MD; David B. Clifford, MD; Houda Hachad, Pharm. D; René H. Levy, Ph. D © 2012 AMERICAN ACADEMY OF NEUROLOGY

The AAN develops these presentation slides as educational tools for neurologists and other health care practitioners. You may download and retain a single copy for your personal use. Please contact guidelines@aan. com to learn about options for sharing this content beyond your personal use. © 2012 AMERICAN ACADEMY OF NEUROLOGY

Presentation Objectives • To present analysis of the evidence for drug–drug interactions between antiepileptic drugs (AEDs) and antiretrovirals (ARVs) • To present evidence-based recommendations © 2012 AMERICAN ACADEMY OF NEUROLOGY

Overview • Background • Gaps in care • American Academy of Neurology (AAN) guideline process • Analysis of evidence, conclusions, recommendations • Recommendations for future research © 2012 AMERICAN ACADEMY OF NEUROLOGY

Background • No formal AED treatment guidelines currently exist for individuals with HIV/AIDS. • Worldwide the concurrent use of AEDs and ARVs is substantial. o Seizure disorders are common in individuals infected with HIV, with a reported incidence as high as 11%. 1– 3 o HIV/AIDS, especially prevalent in sub-Saharan Africa, is becoming a chronic condition as ARV therapies become increasingly available. 4 o The indications for AEDs include neurologic and psychiatric conditions other than epilepsy. © 2012 AMERICAN ACADEMY OF NEUROLOGY

Background, cont. • Potential interactions between ARVs and AEDs are complex and extensive. o P 450 system enzyme induction effects of several oldergeneration AEDs (e. g. , phenobarbital, carbamazepine, phenytoin) are of greatest concern. o P 450 system enzyme inducers might be expected to lower the effective dose of nonnucleotide reverse transcriptase inhibitors (NNRTIs) and protease inhibitors (PIs). • Several potential mechanisms of interaction and the impact of ARVs on AEDs also warrant consideration. o Effective HIV care requires lifelong treatment using regimens typically comprising at least 3 drugs. 5 © 2012 AMERICAN ACADEMY OF NEUROLOGY

Background, cont. • AED-ARV interactions that raise blood levels of drugs in either class may increase toxicity risk. • Use of ARVs that reduce AED levels could lead to loss of therapeutic effects, including seizure control. • Use of AEDs that decrease ARV levels (e. g. , the enzyme -inducing AEDs [EI-AEDs] phenytoin, phenobarbital, and carbamazepine) may lead to virologic failure. o Because first-line AED availability in most developing countries is limited to phenobarbital, carbamazepine, and phenytoin, and ARV regimen options may also be limited, there is substantial risk for occurrence of clinically important drug interactions. 6, 7 © 2012 AMERICAN ACADEMY OF NEUROLOGY

Gaps in Care • No formal AED treatment guidelines currently exist for individuals with HIV/AIDS; at the same time, seizure disorders are common in individuals infected with HIV. • Worldwide the concurrent use of AEDs and ARVs is substantial, as ARV use expands with the increasingly chronic nature of HIV/AIDS and the increased use of AEDs for conditions other than epilepsy (e. g. , neuropathic pain). • Potential interactions between ARVs and AEDs are complex and extensive. This, along with the impact of ARVs on AEDs, warrants consideration. © 2012 AMERICAN ACADEMY OF NEUROLOGY

Gaps in Care, cont. • AED-ARV interactions that raise blood levels of drugs in either class may increase toxicity risk. Use of ARVs that reduce AED levels could lead to loss of therapeutic AED effects, including seizure control. Use of AEDs that decrease ARV levels (e. g. , EI-AEDS phenytoin, phenobarbital, and carbamazepine) may lead to virologic failure and ARV resistant HIV strains. © 2012 AMERICAN ACADEMY OF NEUROLOGY

AAN Guideline Process Clinical Question Evidence Conclusions Recommendations © 2012 AMERICAN ACADEMY OF NEUROLOGY

Clinical Questions • What is the evidence that AED-ARV interactions are clinically meaningful? • What is the evidence for an interaction between AEDs and PI ARVs? • What is the evidence for interaction between AEDs and integrase inhibitors? • What is the evidence for an interaction between AEDs and nucleoside reverse transcriptase inhibitor and NNRTI ARVs? © 2012 AMERICAN ACADEMY OF NEUROLOGY

Literature Search/Review Rigorous, Comprehensive, Transparent Complete Search Review abstracts Review full text Relevant © 2012 AMERICAN ACADEMY OF NEUROLOGY Select articles

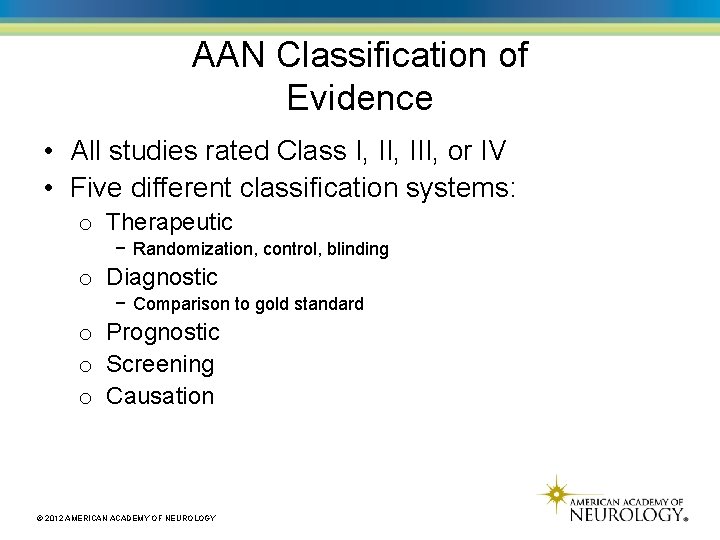
AAN Classification of Evidence • All studies rated Class I, III, or IV • Five different classification systems: o Therapeutic − Randomization, control, blinding o Diagnostic − Comparison to gold standard o Prognostic o Screening o Causation © 2012 AMERICAN ACADEMY OF NEUROLOGY

AAN Level of Recommendations • A = Established as effective, ineffective or harmful (or established as useful/predictive or not useful/predictive) for the given condition in the specified population. • B = Probably effective, ineffective or harmful (or probably useful/predictive or not useful/predictive) for the given condition in the specified population. • C = Possibly effective, ineffective or harmful (or possibly useful/predictive or not useful/predictive) for the given condition in the specified population. • U = Data inadequate or conflicting; given current knowledge, treatment (test, predictor) is unproven. Note that recommendations can be positive or negative. © 2012 AMERICAN ACADEMY OF NEUROLOGY

Translating Class to Recommendations • A = Requires at least two consistent Class I studies. * • B = Requires at least one Class I study or two consistent Class II studies. • C = Requires at least one Class II study or two consistent Class III studies. • U = Studies not meeting criteria for Class I through Class III. © 2012 AMERICAN ACADEMY OF NEUROLOGY

Translating Class to Recommendations, cont. *In exceptional cases, one convincing Class I study may suffice for an “A” recommendation if 1) all criteria are met, 2) the magnitude of effect is large (relative rate improved outcome >5 and the lower limit of the confidence interval is >2). © 2012 AMERICAN ACADEMY OF NEUROLOGY

Applying This Process to the Issue We will now turn our attention to the guidelines. © 2012 AMERICAN ACADEMY OF NEUROLOGY

Methods • MEDLINE o 1950 to April 2008 (updated in 2010) o Relevant, fully published, peer-reviewed articles o For the original and updated search strategies, see appendices e-1 and e-2 of the published guideline © 2012 AMERICAN ACADEMY OF NEUROLOGY

Methods, cont. • At least two authors reviewed each article for inclusion. • Risk of bias was determined using the classification of evidence for each study (Classes I–IV). • Strength of practice recommendations were linked directly to levels of evidence (Levels A, B, C, and U). • Conflicts of interest were disclosed. © 2012 AMERICAN ACADEMY OF NEUROLOGY

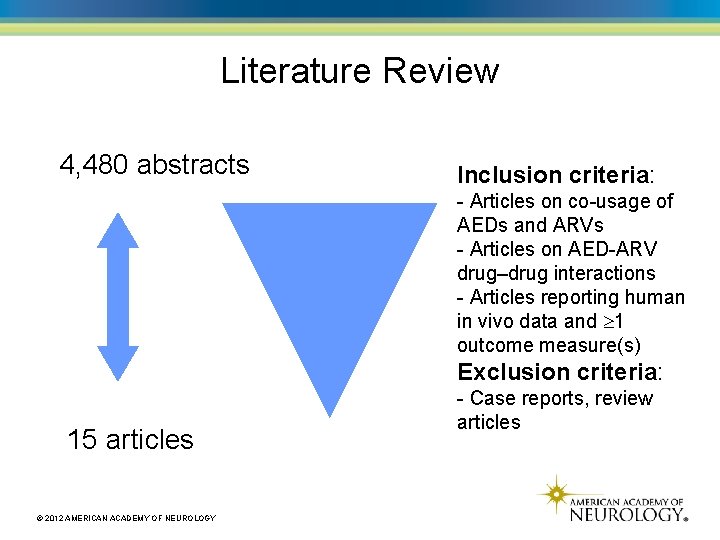
Literature Review 4, 480 abstracts Inclusion criteria: - Articles on co-usage of AEDs and ARVs - Articles on AED-ARV drug–drug interactions - Articles reporting human in vivo data and 1 outcome measure(s) Exclusion criteria: 15 articles © 2012 AMERICAN ACADEMY OF NEUROLOGY - Case reports, review articles

AAN Classification of Evidence for Therapeutic Intervention • Class I: A randomized, controlled clinical trial of the intervention of interest with masked or objective outcome assessment, in a representative population. Relevant baseline characteristics are presented and substantially equivalent among treatment groups or there is appropriate statistical adjustment for differences. The following are also required: o Concealed allocation o Primary outcome(s) clearly defined o Exclusion/inclusion criteria clearly defined © 2012 AMERICAN ACADEMY OF NEUROLOGY

AAN Classification of Evidence for Therapeutic Intervention, cont. o Adequate accounting for dropouts (with at least 80% of enrolled subjects completing the study) and crossovers with numbers sufficiently low to have minimal potential for bias. o For noninferiority or equivalence trials claiming to prove efficacy for one or both drugs, the following are also required*: – The authors explicitly state the clinically meaningful difference to be excluded by defining the threshold for equivalence or noninferiority. – The standard treatment used in the study is substantially similar to that used in previous studies establishing efficacy of the standard treatment (e. g. , for a drug, the mode of administration, dose and dosage adjustments are similar to those previously shown to be effective). – The inclusion and exclusion criteria for patient selection and the outcomes of patients on the standard treatment are comparable to those of previous studies establishing efficacy of the standard treatment. – The interpretation of the results of the study is based upon a per protocol analysis that takes into account dropouts or crossovers. © 2012 AMERICAN ACADEMY OF NEUROLOGY

AAN Classification of Evidence for Therapeutic Intervention, cont. • Class II: A randomized controlled clinical trial of the intervention of interest in a representative population with masked or objective outcome assessment that lacks one criteria a e above or a prospective matched cohort study with masked or objective outcome assessment in a representative population that meets b e above. Relevant baseline characteristics are presented and substantially equivalent among treatment groups or there is appropriate statistical adjustment for differences. • Class III: All other controlled trials (including well-defined natural history controls or patients serving as own controls) in a representative population, where outcome is independently assessed, or independently derived by objective outcome measurement. ** © 2012 AMERICAN ACADEMY OF NEUROLOGY

AAN Classification of Evidence for Therapeutic Intervention, cont. • Class IV: Studies not meeting Class I, II or III criteria including consensus or expert opinion. *Note that numbers 1 3 in Class I, item 5 are required for Class II in equivalence trials. If any one of the three is missing, the class is automatically downgraded to Class III. **Objective outcome measurement: an outcome measure that is unlikely to be affected by an observer’s (patient, treating physician, investigator) expectation or bias (e. g. , blood tests, administrative outcome data). © 2012 AMERICAN ACADEMY OF NEUROLOGY

Clinical Question 1: What is the evidence that AED -ARV interactions are clinically meaningful? © 2012 AMERICAN ACADEMY OF NEUROLOGY

ARVs and EI-AEDs Conclusion: • Coadministration of highly active ARV therapy containing a PI or NNRTI and an EI-AED possibly results in higher virologic failure rates (1 Class II study). Recommendation: • It may be important to avoid EI-AEDs in people on ARV regimens that include PIs or NNRTIs, as pharmacokinetic interactions may result in virologic failure, which has clinical implications for disease progression and development of ARV resistance. If such regimens are required for seizure control, patients may be monitored through pharmacokinetic assessments to ensure efficacy of the ARV regimen (Level C). © 2012 AMERICAN ACADEMY OF NEUROLOGY

Clinical Question 2: What is the evidence for an interaction between AEDs and PI ARVs? © 2012 AMERICAN ACADEMY OF NEUROLOGY

Phenytoin: Impact on Lopinavir/ritonavir Conclusion: • Phenytoin possibly reduces lopinavir and ritonavir levels by about 30% (1 Class II study). Recommendation: • Patients receiving phenytoin may require a lopinavir/ritonavir dosage increase of about 50% to maintain unchanged serum concentrations (Level C). © 2012 AMERICAN ACADEMY OF NEUROLOGY

Atazanavir and Atazanavir/ritonavir: Impact on Lamotrigine Conclusion: • Ritonavir/atazanavir possibly reduces lamotrigine exposure by about 30% (1 Class II study). Recommendation: • Coadministration of atazanavir and lamotrigine may not require lamotrigine dosage adjustment (Level C). • Patients receiving ritonavir/atazanavir may require a lamotrigine dosage increase of about 50% to maintain unchanged lamotrigine serum concentrations (Level C). © 2012 AMERICAN ACADEMY OF NEUROLOGY

Clinical Question 3: What is the evidence for interaction between AEDs and integrase inhibitors? © 2012 AMERICAN ACADEMY OF NEUROLOGY

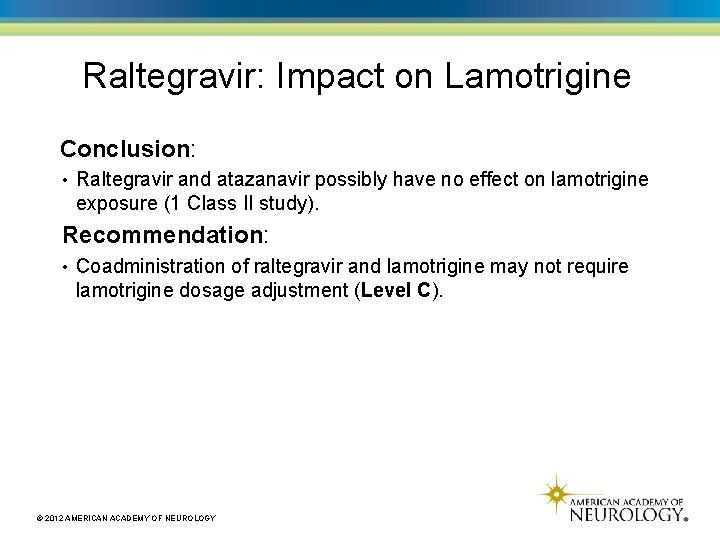
Raltegravir: Impact on Lamotrigine Conclusion: • Raltegravir and atazanavir possibly have no effect on lamotrigine exposure (1 Class II study). Recommendation: • Coadministration of raltegravir and lamotrigine may not require lamotrigine dosage adjustment (Level C). © 2012 AMERICAN ACADEMY OF NEUROLOGY

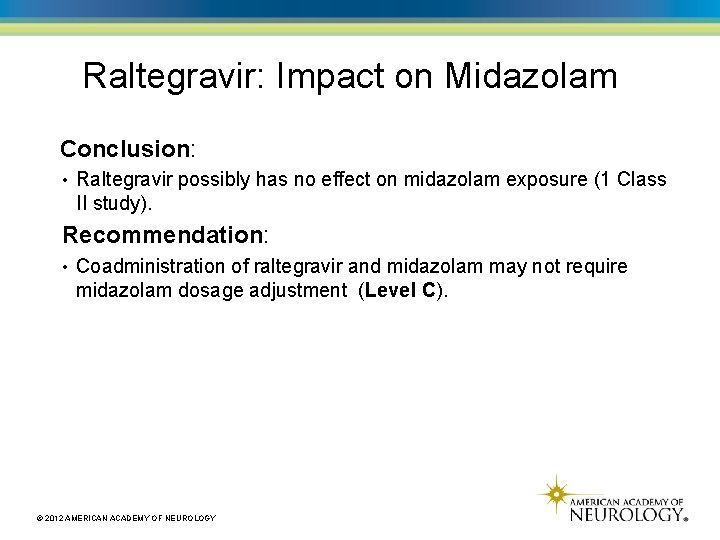
Raltegravir: Impact on Midazolam Conclusion: • Raltegravir possibly has no effect on midazolam exposure (1 Class II study). Recommendation: • Coadministration of raltegravir and midazolam may not require midazolam dosage adjustment (Level C). © 2012 AMERICAN ACADEMY OF NEUROLOGY

Clinical Question 4: What is the evidence for an interaction between AEDs and nucleoside reverse transcriptase inhibitor and NNRTI ARVs? © 2012 AMERICAN ACADEMY OF NEUROLOGY

Valproic Acid: Impact on Efavirenz / Efavirenz: Impact on Valproic Acid Conclusion: • Valproic acid possibly has no effect on efavirenz exposure (Class II study). Recommendation: • Coadministration of valproic acid and efavirenz may not require efavirenz dosage adjustment (Level C). © 2012 AMERICAN ACADEMY OF NEUROLOGY

Valproic Acid: Impact on Zidovudine Conclusion: • Valproic acid possibly increases zidovudine exposure (1 Class II study). Recommendation: • Patients receiving valproic acid may require a zidovudine dosage reduction to maintain unchanged serum zidovudine concentrations (Level C). © 2012 AMERICAN ACADEMY OF NEUROLOGY

Effects of Combining Other AEDs and ARVs Conclusion: • The evidence is insufficient to support or refute other pharmacokinetic AED-ARV interactions (single Class III/multiple Class IV studies). Recommendation: • Patients may be counseled that it is unclear whether dosage adjustment is necessary when other AEDs and ARVs are combined (Level U). © 2012 AMERICAN ACADEMY OF NEUROLOGY

Clinical Context • A retrospective cohort study and numerous pharmacokinetic studies indicate that EI-AEDs interact with ARVs. • The optimal choice of epilepsy treatment in patients with HIV should reflect an accounting for the metabolic and inhibitory/inducing profiles of coadministered drugs. © 2012 AMERICAN ACADEMY OF NEUROLOGY

Clinical Context, cont. • Clinicians who prescribe ARVs and AEDs are encouraged to refer to the Department of Health and Human Services treatment guidelines for HIV/AIDS, which provide specific recommendations for the management of possible drug– drug interactions with AED-ARV combinations (available at http: //aidsinfo. nih. gov/contentfiles/Adultand. Adolescent. GL. p df). • For newer ARV agents, minimal data exist on drug interactions with AEDs. © 2012 AMERICAN ACADEMY OF NEUROLOGY

Future Research • Future research regarding AED-ARV interactions is needed. • Special priority should be given to the study of first-line AED-ARV combinations used in low- and middle-income countries where second-line agents may not be available. © 2012 AMERICAN ACADEMY OF NEUROLOGY

References 1. 2. 3. 4. 5. 6. 7. Holmberg SD, Buchbinder SP, Conley LJ, et al. The spectrum of medical conditions and symptoms before acquired immunodeficiency syndrome in homosexual and bisexual men infected with the human immunodeficiency virus. Am J Epidemiol 1995; 141: 395– 404; discussion 405– 396. Kellinghaus C, Engbring C, Kovac S, et al. Frequency of seizures and epilepsy in neurological HIV-infected patients. Seizure 2008; 17: 27– 33. Wong MC, Suite ND, Labar DR. Seizures in human immunodeficiency virus infection. Arch Neurol 1990; 47: 640– 642. Bradshaw D, Groenewald P, Laubscher R, et al. Initial burden of disease estimates for South Africa, 2000. S Afr Med J 2003; 93: 682– 688. World Health Organization. Patient Monitoring Guidelines for HIV Care and Antiretroviral Therapy. Geneva: WHO Press; 2006. Epilepsy and HIV: a dangerous combination. Lancet Neurol 2007; 6: 747. Birbeck G, Chomba E, Ddumba E, Kauye F, Mielke J. Lack of appropriate treatment for people with comorbid HIV/AIDS and epilepsy in sub-Saharan Africa. Epilepsia 2007; 48: 1424– 1425. © 2012 AMERICAN ACADEMY OF NEUROLOGY

References, cont. For a complete list of references, please access the full guideline at www. aan. com/guidelines © 2012 AMERICAN ACADEMY OF NEUROLOGY

Questions/Comments © 2012 AMERICAN ACADEMY OF NEUROLOGY

Thank you for your participation! © 2012 AMERICAN ACADEMY OF NEUROLOGY
 Antiepileptic drug classification
Antiepileptic drug classification Antiepileptic side effects
Antiepileptic side effects Sodium valproate mechanism of action
Sodium valproate mechanism of action Antiepileptic side effects
Antiepileptic side effects Phenytoin mechanism of action
Phenytoin mechanism of action Fycompa moa
Fycompa moa Antiepileptic drugs classification
Antiepileptic drugs classification Methods of adulteration of crude drugs
Methods of adulteration of crude drugs Natural selection and drug resistance
Natural selection and drug resistance Natural selection
Natural selection Balancing selection vs stabilizing selection
Balancing selection vs stabilizing selection Similarities
Similarities K selected
K selected Natural selection vs artificial selection
Natural selection vs artificial selection Difference between continuous and discontinuous variation
Difference between continuous and discontinuous variation Disruptive selextion
Disruptive selextion Clumped dispersion
Clumped dispersion Natural selection vs artificial selection
Natural selection vs artificial selection Two way selection and multiway selection
Two way selection and multiway selection Multiway selection in c
Multiway selection in c Procedure of pure line selection
Procedure of pure line selection Selection of an appropriate project approach
Selection of an appropriate project approach Fspos vägledning för kontinuitetshantering
Fspos vägledning för kontinuitetshantering Typiska novell drag
Typiska novell drag Nationell inriktning för artificiell intelligens
Nationell inriktning för artificiell intelligens Vad står k.r.å.k.a.n för
Vad står k.r.å.k.a.n för Shingelfrisyren
Shingelfrisyren En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Kassaregister ideell förening
Kassaregister ideell förening Personlig tidbok fylla i
Personlig tidbok fylla i Sura för anatom
Sura för anatom Vad är densitet
Vad är densitet Datorkunskap för nybörjare
Datorkunskap för nybörjare Stig kerman
Stig kerman Att skriva en debattartikel
Att skriva en debattartikel Delegerande ledarstil
Delegerande ledarstil Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Påbyggnader för flakfordon
Påbyggnader för flakfordon Vätsketryck formel
Vätsketryck formel Svenskt ramverk för digital samverkan
Svenskt ramverk för digital samverkan Jag har gått inunder stjärnor text
Jag har gått inunder stjärnor text Presentera för publik crossboss
Presentera för publik crossboss Jiddisch
Jiddisch Kanaans land
Kanaans land



































































