ADAPTDES OneYear Results Assessment of Dual Anti Platelet



![ADAPT-DES: Mortality according to post-PCI PRU 5 HR [95%CI] = 1. 62 [1. 18, ADAPT-DES: Mortality according to post-PCI PRU 5 HR [95%CI] = 1. 62 [1. 18,](https://slidetodoc.com/presentation_image_h/ca3be09165022a1389a2ff0627b77e62/image-16.jpg)

- Slides: 24

ADAPT-DES One-Year Results Assessment of Dual Anti. Platelet Therapy with Drug-Eluting Stents A Large-Scale, Multicenter, Prospective, Observational Study of the Impact of Clopidogrel and Aspirin Hyporesponsiveness on Patient Outcomes Gregg W. Stone, MD Columbia University Medical Center New. York-Presbyterian Hospital Cardiovascular Research Foundation

Disclosures • Gregg W. Stone ¡ Consultant to Eli Lilly, Daiichi Sankyo, Astra. Zeneca, Medtronic, Boston Scientific, Abbott Vascular, Volcano, The Medicines Company

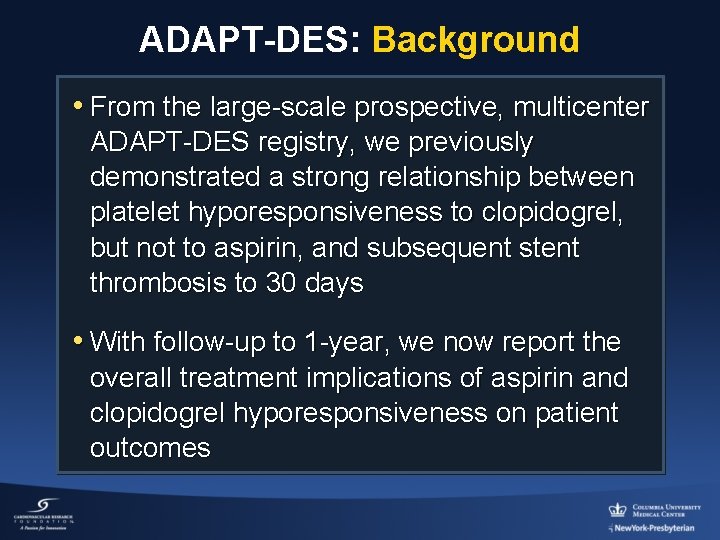
ADAPT-DES: Background • From the large-scale prospective, multicenter ADAPT-DES registry, we previously demonstrated a strong relationship between platelet hyporesponsiveness to clopidogrel, but not to aspirin, and subsequent stent thrombosis to 30 days • With follow-up to 1 -year, we now report the overall treatment implications of aspirin and clopidogrel hyporesponsiveness on patient outcomes

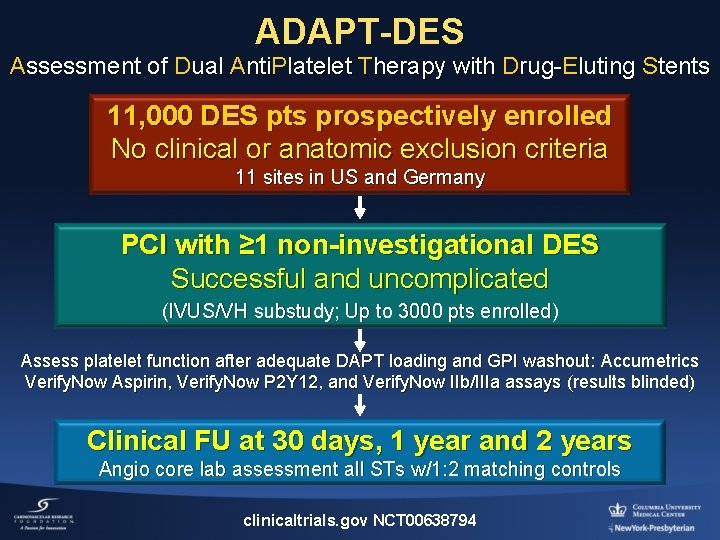
ADAPT-DES Assessment of Dual Anti. Platelet Therapy with Drug-Eluting Stents 11, 000 DES pts prospectively enrolled No clinical or anatomic exclusion criteria 11 sites in US and Germany PCI with ≥ 1 non-investigational DES Successful and uncomplicated (IVUS/VH substudy; Up to 3000 pts enrolled) Assess platelet function after adequate DAPT loading and GPI washout: Accumetrics Verify. Now Aspirin, Verify. Now P 2 Y 12, and Verify. Now IIb/IIIa assays (results blinded) Clinical FU at 30 days, 1 year and 2 years Angio core lab assessment all STs w/1: 2 matching controls clinicaltrials. gov NCT 00638794

ADAPT-DES: Study organization Principal investigator: Gregg W. Stone (& Chuck Simonton prior to joining AVD) Co-principal investigators: Thomas Stuckey, Bruce Brodie, Mike Rinaldi Pharmacology committee: Paul Gurbel and Steve Steinhubl Sponsor (IDE): Cardiovascular Research Foundation Site management & monitoring: R. Stuart Dickson Institute For Health Studies Michael Dulin, director, Sherry Laurent, consultant Data management: R. Stuart Dickson Institute For Health Studies Susan Christopher, project lead Event adjudication: Cardiovascular Research Foundation Roxana Mehran and Ecaterina Cristea, directors Angio and IVUS core labs: Cardiovascular Research Foundation Ecaterina Cristea and Akiko Maehara, directors Biostatistics: Cardiovascular Research Foundation Helen Parise, director Financial support: Boston Scientific, Abbott Vascular, Medtronic, Cordis, Biosensors, The Medicines Company, Daiichi-Sankyo, Eli Lilly, Volcano, Accumetrics

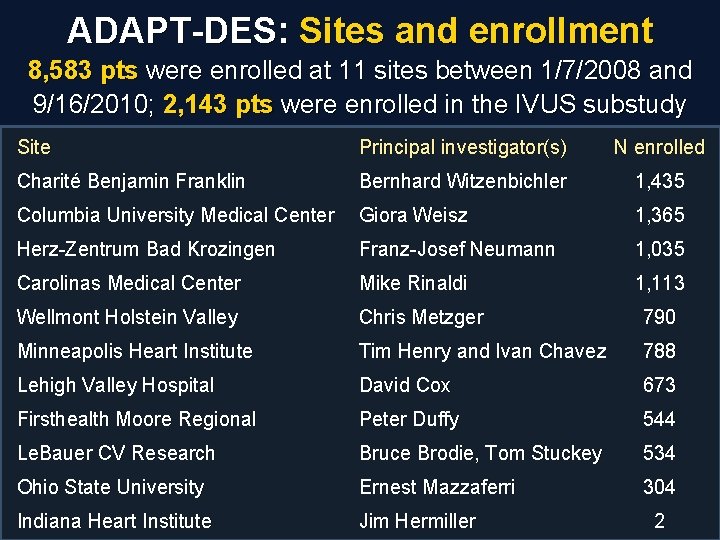
ADAPT-DES: Sites and enrollment 8, 583 pts were enrolled at 11 sites between 1/7/2008 and 9/16/2010; 2, 143 pts were enrolled in the IVUS substudy Site Principal investigator(s) N enrolled Charité Benjamin Franklin Bernhard Witzenbichler 1, 435 Columbia University Medical Center Giora Weisz 1, 365 Herz-Zentrum Bad Krozingen Franz-Josef Neumann 1, 035 Carolinas Medical Center Mike Rinaldi 1, 113 Wellmont Holstein Valley Chris Metzger 790 Minneapolis Heart Institute Tim Henry and Ivan Chavez 788 Lehigh Valley Hospital David Cox 673 Firsthealth Moore Regional Peter Duffy 544 Le. Bauer CV Research Bruce Brodie, Tom Stuckey 534 Ohio State University Ernest Mazzaferri 304 Indiana Heart Institute Jim Hermiller 2

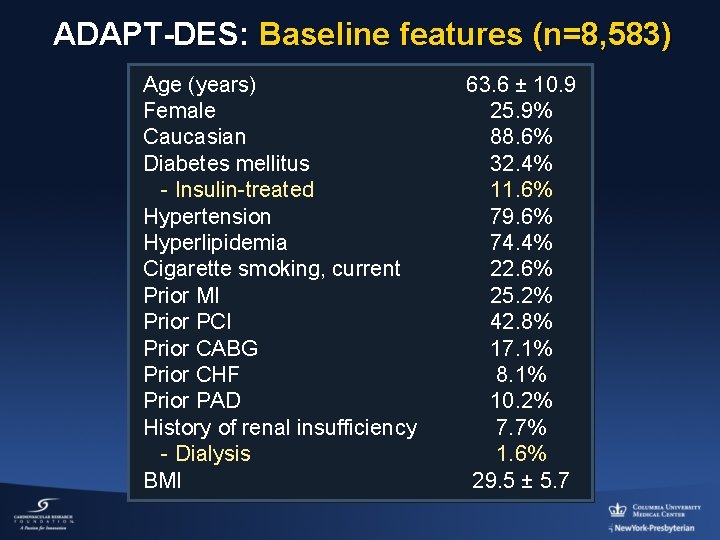
ADAPT-DES: Baseline features (n=8, 583) Age (years) Female Caucasian Diabetes mellitus - Insulin-treated Hypertension Hyperlipidemia Cigarette smoking, current Prior MI Prior PCI Prior CABG Prior CHF Prior PAD History of renal insufficiency - Dialysis BMI 63. 6 ± 10. 9 25. 9% 88. 6% 32. 4% 11. 6% 79. 6% 74. 4% 22. 6% 25. 2% 42. 8% 17. 1% 8. 1% 10. 2% 7. 7% 1. 6% 29. 5 ± 5. 7

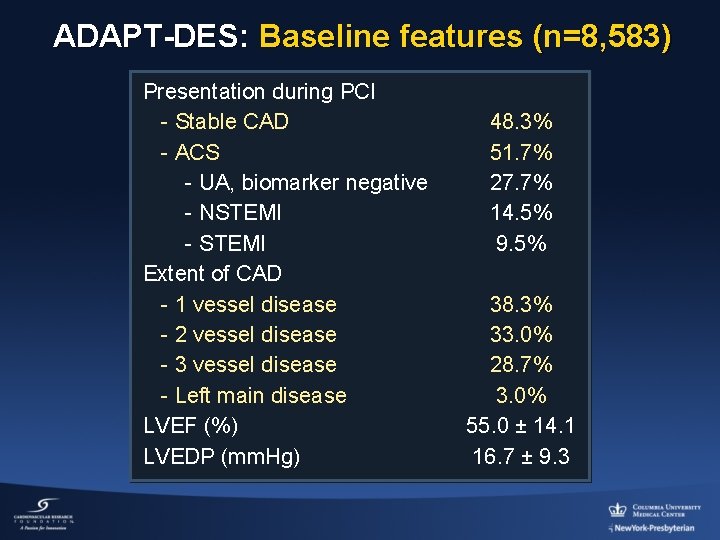
ADAPT-DES: Baseline features (n=8, 583) Presentation during PCI - Stable CAD - ACS - UA, biomarker negative - NSTEMI - STEMI Extent of CAD - 1 vessel disease - 2 vessel disease - 3 vessel disease - Left main disease LVEF (%) LVEDP (mm. Hg) 48. 3% 51. 7% 27. 7% 14. 5% 9. 5% 38. 3% 33. 0% 28. 7% 3. 0% 55. 0 ± 14. 1 16. 7 ± 9. 3

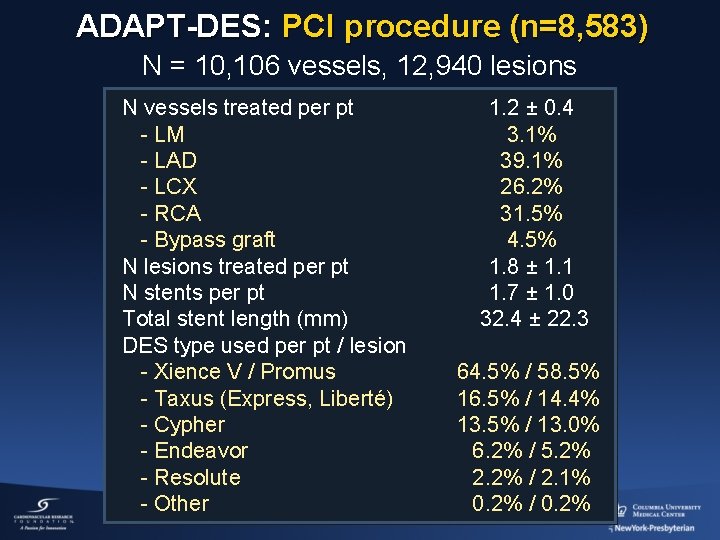
ADAPT-DES: PCI procedure (n=8, 583) N = 10, 106 vessels, 12, 940 lesions N vessels treated per pt - LM - LAD - LCX - RCA - Bypass graft N lesions treated per pt N stents per pt Total stent length (mm) DES type used per pt / lesion - Xience V / Promus - Taxus (Express, Liberté) - Cypher - Endeavor - Resolute - Other 1. 2 ± 0. 4 3. 1% 39. 1% 26. 2% 31. 5% 4. 5% 1. 8 ± 1. 1 1. 7 ± 1. 0 32. 4 ± 22. 3 64. 5% / 58. 5% 16. 5% / 14. 4% 13. 5% / 13. 0% 6. 2% / 5. 2% 2. 2% / 2. 1% 0. 2% / 0. 2%

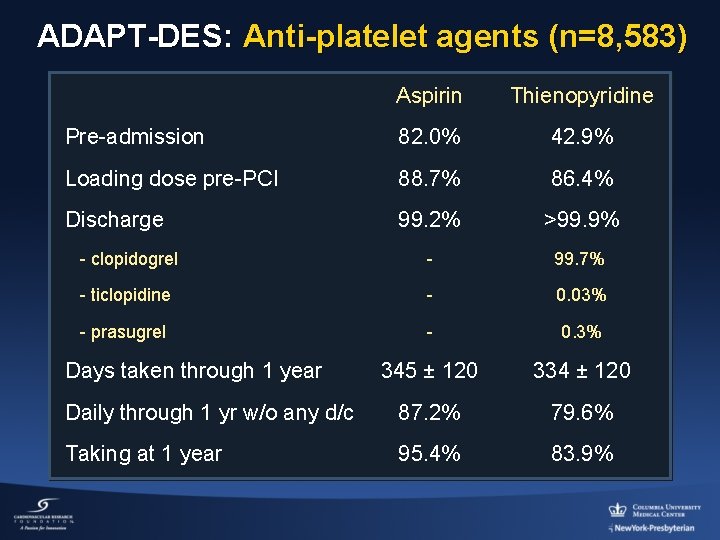
ADAPT-DES: Anti-platelet agents (n=8, 583) Aspirin Thienopyridine Pre-admission 82. 0% 42. 9% Loading dose pre-PCI 88. 7% 86. 4% Discharge 99. 2% >99. 9% - clopidogrel - 99. 7% - ticlopidine - 0. 03% - prasugrel - 0. 3% 345 ± 120 334 ± 120 Daily through 1 yr w/o any d/c 87. 2% 79. 6% Taking at 1 year 95. 4% 83. 9% Days taken through 1 year

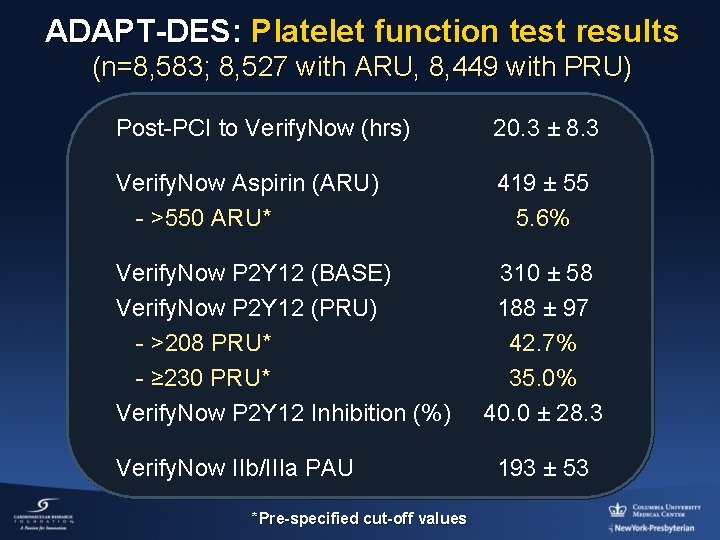
ADAPT-DES: Platelet function test results (n=8, 583; 8, 527 with ARU, 8, 449 with PRU) Post-PCI to Verify. Now (hrs) 20. 3 ± 8. 3 Verify. Now Aspirin (ARU) - >550 ARU* 419 ± 55 5. 6% Verify. Now P 2 Y 12 (BASE) Verify. Now P 2 Y 12 (PRU) - >208 PRU* - ≥ 230 PRU* Verify. Now P 2 Y 12 Inhibition (%) Verify. Now IIb/IIIa PAU *Pre-specified cut-off values 310 ± 58 188 ± 97 42. 7% 35. 0% 40. 0 ± 28. 3 193 ± 53

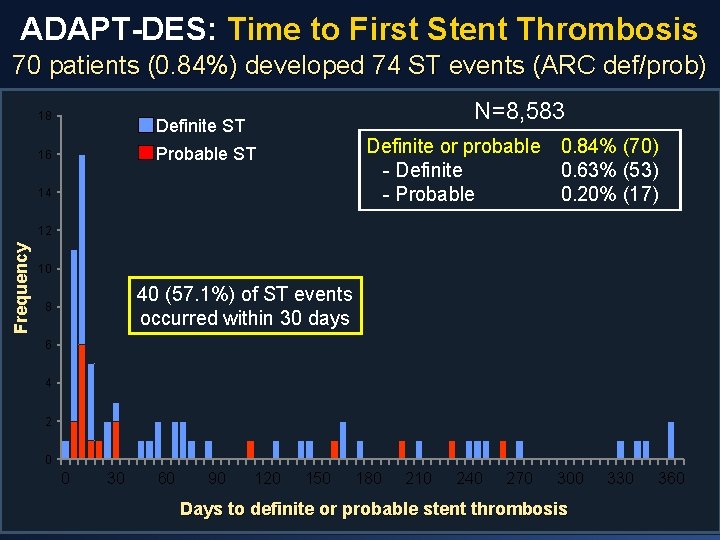
ADAPT-DES: Time to First Stent Thrombosis 70 patients (0. 84%) developed 74 ST events (ARC def/prob) 18 N=8, 583 Definite ST Definite or probable 0. 84% (70) - Definite 0. 63% (53) - Probable 0. 20% (17) Probable ST 16 14 Frequency 12 10 40 (57. 1%) of ST events occurred within 30 days 8 6 4 2 0 0 30 60 90 120 150 180 210 240 270 300 Days to definite or probable stent thrombosis 330 360

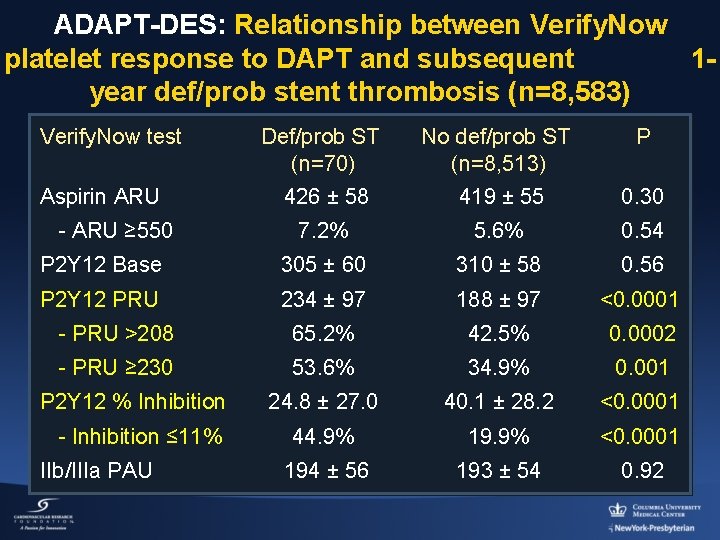
ADAPT-DES: Relationship between Verify. Now platelet response to DAPT and subsequent 1 year def/prob stent thrombosis (n=8, 583) Verify. Now test Def/prob ST (n=70) 426 ± 58 No def/prob ST (n=8, 513) 419 ± 55 0. 30 7. 2% 5. 6% 0. 54 P 2 Y 12 Base 305 ± 60 310 ± 58 0. 56 P 2 Y 12 PRU 234 ± 97 188 ± 97 <0. 0001 - PRU >208 65. 2% 42. 5% 0. 0002 - PRU ≥ 230 53. 6% 34. 9% 0. 001 24. 8 ± 27. 0 40. 1 ± 28. 2 <0. 0001 44. 9% 19. 9% <0. 0001 194 ± 56 193 ± 54 0. 92 Aspirin ARU - ARU ≥ 550 P 2 Y 12 % Inhibition - Inhibition ≤ 11% IIb/IIIa PAU P

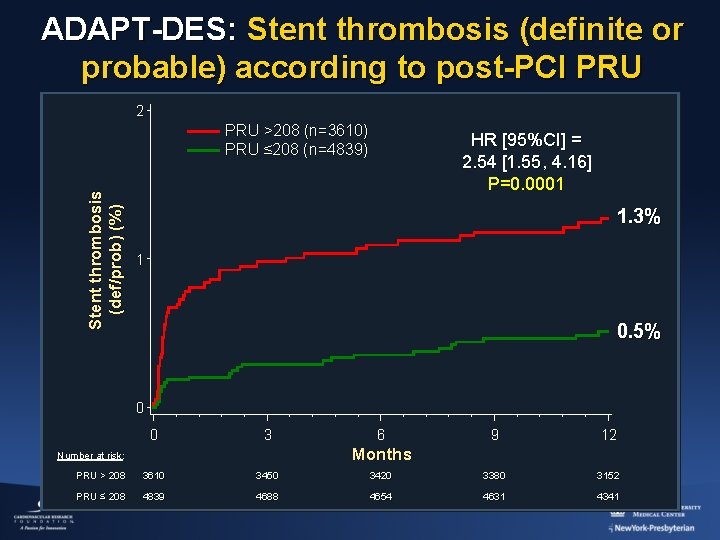
ADAPT-DES: Stent thrombosis (definite or probable) according to post-PCI PRU 2 Stent thrombosis (def/prob) (%) PRU >208 (n=3610) PRU ≤ 208 (n=4839) HR [95%CI] = 2. 54 [1. 55, 4. 16] P=0. 0001 1. 3% 1 0. 5% 0 0 3 6 9 12 Months Number at risk: PRU > 208 3610 3450 3420 3380 3152 PRU ≤ 208 4839 4688 4654 4631 4341

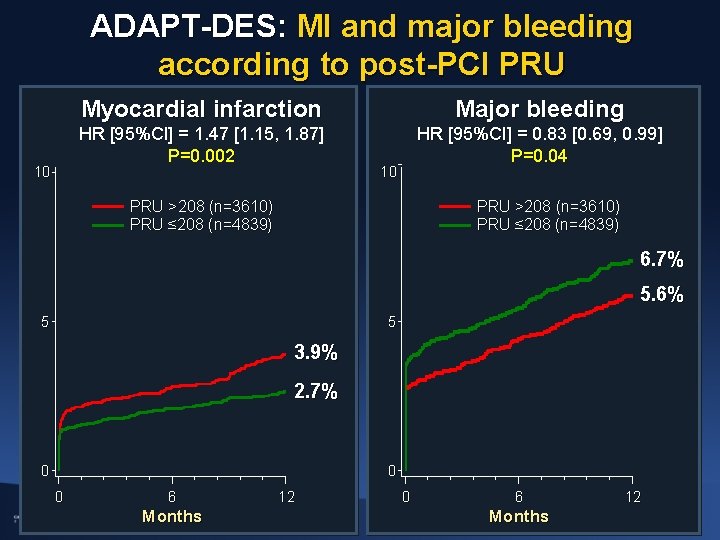
ADAPT-DES: MI and major bleeding according to post-PCI PRU 10 Myocardial infarction Major bleeding HR [95%CI] = 1. 47 [1. 15, 1. 87] P=0. 002 HR [95%CI] = 0. 83 [0. 69, 0. 99] P=0. 04 10 PRU >208 (n=3610) PRU ≤ 208 (n=4839) 6. 7% 5. 6% 5 5 3. 9% 2. 7% 0 0 0 6 Months 12
![ADAPTDES Mortality according to postPCI PRU 5 HR 95CI 1 62 1 18 ADAPT-DES: Mortality according to post-PCI PRU 5 HR [95%CI] = 1. 62 [1. 18,](https://slidetodoc.com/presentation_image_h/ca3be09165022a1389a2ff0627b77e62/image-16.jpg)
ADAPT-DES: Mortality according to post-PCI PRU 5 HR [95%CI] = 1. 62 [1. 18, 2. 22] P=0. 002 PRU >208 (n=3610) PRU ≤ 208 (n=4839) Mortality (%) 4 3 2. 4% 2 1. 5% 1 0 0 3 6 9 12 Months Number at risk: PRU > 208 3610 3475 3447 3408 3181 PRU ≤ 208 4839 4696 4664 4645 4365

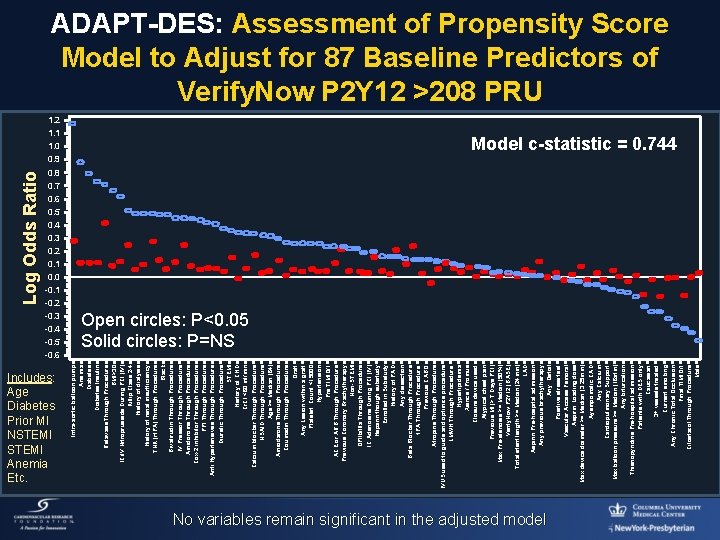
1. 2 1. 1 1. 0 0. 9 0. 8 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0. 0 -0. 1 -0. 2 -0. 3 -0. 4 -0. 5 -0. 6 Includes: Age Diabetes Prior MI NSTEMI Anemia Etc. Intra-aortic balloon pump Anemia Diabetes Insulin Retavase Through Procedure BMI>30 IC/IV Nitroprusside During PCI (IV) Killip Class 2 -4 History of dialysis History of renal insufficiency TNK (rt. PA) Through Procedure Black Bivalirudin Through Procedure IV Pressor Through Procedure Amidorome Through Procedure Cox-2 inhibitor Through Procedure PPI Through Procedure Anti Hypertensives Through Procedure Diuretic Through Procedure STEMI History of CHD Cr. Cl <60 ml/min Calcium blocker Through Procedure NSAID Through Procedure Age >= Median (64) Amiodarone Through Procedure Coumadin Through Procedure Graft Any Lesion within a graft Platelet Count <15000 Hypertension Pre TIMI 0/1 ACE or ARB Through Procedure Previous Coronary Brachytherapy Non-STEMI GPIIb/IIIa Through Procedure IC Adenosine During PCI (IV) Heparin though substudy Enrolled in Substudy History of PAD Any dissection Beta Blocker Through Procedure t. PA Through Procedure Previous CABG Atropine Through Procedure IVUS used to guide and optimize procedure LMWH Through Procedure Hyperlipidemia Xience / Promus Closure device used Atypical chest pain Previous MI (> 7 Days PCI) Max Pre-stenosis >= Median (90%) Verify. Now P 2 Y 12 (BASE) Total stent length >= Median (24 mm) LAD Aspirin Pre-hospital admission Any previous brachytherapy Any Ostial Positive stress test Vascular Access Femoral Aspirin Loading dose Max device diameter >= Median (3. 25 mm) Aysmpomatic CAD Any Calcium Cardiopulmonary Support Max balloon pressure >= Median (16 atm) Any bifurcation Thienopyridine Pre-hospital admission Patients with DES only Caucasian 3+ vessels treated Current smoking Any Chronic Total Occlusion Final TIMI 0/1 Cilostazol Through Procedure Male Log Odds Ratio ADAPT-DES: Assessment of Propensity Score Model to Adjust for 87 Baseline Predictors of Verify. Now P 2 Y 12 >208 PRU Model c-statistic = 0. 744 Open circles: P<0. 05 Solid circles: P=NS No variables remain significant in the adjusted model

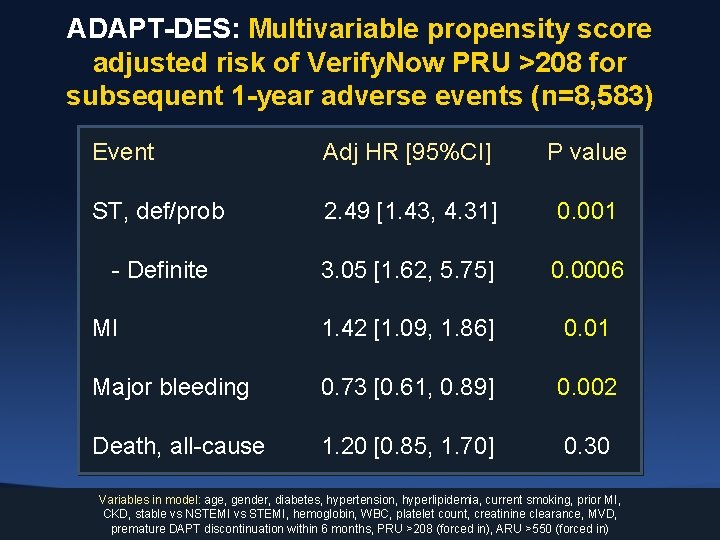
ADAPT-DES: Multivariable propensity score adjusted risk of Verify. Now PRU >208 for subsequent 1 -year adverse events (n=8, 583) Event Adj HR [95%CI] P value ST, def/prob 2. 49 [1. 43, 4. 31] 0. 001 - Definite 3. 05 [1. 62, 5. 75] 0. 0006 MI 1. 42 [1. 09, 1. 86] 0. 01 Major bleeding 0. 73 [0. 61, 0. 89] 0. 002 Death, all-cause 1. 20 [0. 85, 1. 70] 0. 30 Variables in model: age, gender, diabetes, hypertension, hyperlipidemia, current smoking, prior MI, CKD, stable vs NSTEMI vs STEMI, hemoglobin, WBC, platelet count, creatinine clearance, MVD, premature DAPT discontinuation within 6 months, PRU >208 (forced in), ARU >550 (forced in)

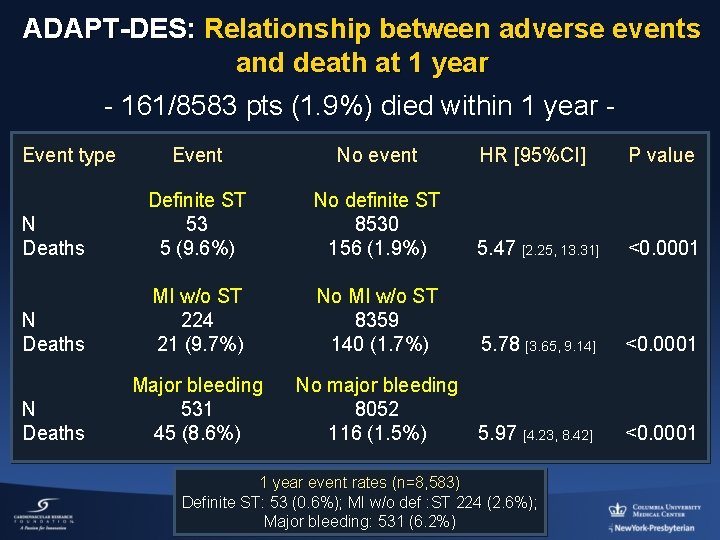
ADAPT-DES: Relationship between adverse events and death at 1 year - 161/8583 pts (1. 9%) died within 1 year Event type Event No event HR [95%CI] P value N Deaths Definite ST 53 5 (9. 6%) No definite ST 8530 156 (1. 9%) 5. 47 [2. 25, 13. 31] <0. 0001 N Deaths MI w/o ST 224 21 (9. 7%) No MI w/o ST 8359 140 (1. 7%) 5. 78 [3. 65, 9. 14] <0. 0001 N Deaths Major bleeding 531 45 (8. 6%) No major bleeding 8052 116 (1. 5%) 5. 97 [4. 23, 8. 42] <0. 0001 1 year event rates (n=8, 583) Definite ST: 53 (0. 6%); MI w/o def : ST 224 (2. 6%); Major bleeding: 531 (6. 2%)

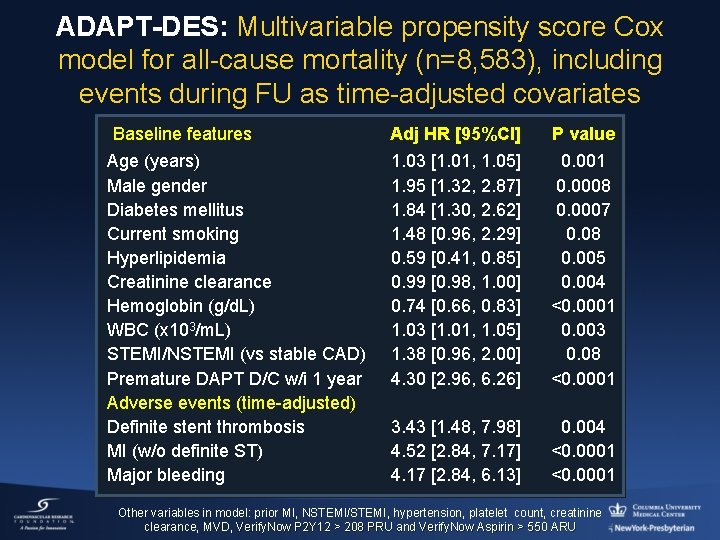
ADAPT-DES: Multivariable propensity score Cox model for all-cause mortality (n=8, 583), including events during FU as time-adjusted covariates Baseline features Age (years) Male gender Diabetes mellitus Current smoking Hyperlipidemia Creatinine clearance Hemoglobin (g/d. L) WBC (x 103/m. L) STEMI/NSTEMI (vs stable CAD) Premature DAPT D/C w/i 1 year Adverse events (time-adjusted) Definite stent thrombosis MI (w/o definite ST) Major bleeding Adj HR [95%CI] P value 1. 03 [1. 01, 1. 05] 1. 95 [1. 32, 2. 87] 1. 84 [1. 30, 2. 62] 1. 48 [0. 96, 2. 29] 0. 59 [0. 41, 0. 85] 0. 99 [0. 98, 1. 00] 0. 74 [0. 66, 0. 83] 1. 03 [1. 01, 1. 05] 1. 38 [0. 96, 2. 00] 4. 30 [2. 96, 6. 26] 0. 001 0. 0008 0. 0007 0. 08 0. 005 0. 004 <0. 0001 0. 003 0. 08 <0. 0001 3. 43 [1. 48, 7. 98] 4. 52 [2. 84, 7. 17] 4. 17 [2. 84, 6. 13] 0. 004 <0. 0001 Other variables in model: prior MI, NSTEMI/STEMI, hypertension, platelet count, creatinine clearance, MVD, Verify. Now P 2 Y 12 > 208 PRU and Verify. Now Aspirin > 550 ARU

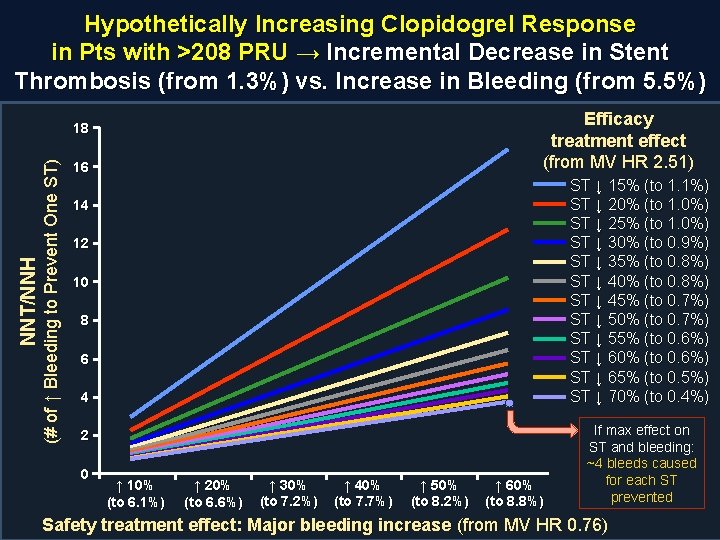
Hypothetically Increasing Clopidogrel Response in Pts with >208 PRU → Incremental Decrease in Stent Thrombosis (from 1. 3%) vs. Increase in Bleeding (from 5. 5%) Efficacy treatment effect (from MV HR 2. 51) (# of ↑ Bleeding to Prevent One ST) NNT/NNH 18 16 ST ↓ 15% (to 1. 1%) ST ↓ 20% (to 1. 0%) ST ↓ 25% (to 1. 0%) ST ↓ 30% (to 0. 9%) ST ↓ 35% (to 0. 8%) ST ↓ 40% (to 0. 8%) ST ↓ 45% (to 0. 7%) ST ↓ 50% (to 0. 7%) ST ↓ 55% (to 0. 6%) ST ↓ 60% (to 0. 6%) ST ↓ 65% (to 0. 5%) ST ↓ 70% (to 0. 4%) 14 12 10 8 6 4 2 0 ↑ 10% (to 6. 1%) ↑ 20% (to 6. 6%) ↑ 30% (to 7. 2%) ↑ 40% (to 7. 7%) ↑ 50% (to 8. 2%) ↑ 60% (to 8. 8%) If max effect on ST and bleeding: ~4 bleeds caused for each ST prevented Safety treatment effect: Major bleeding increase (from MV HR 0. 76)

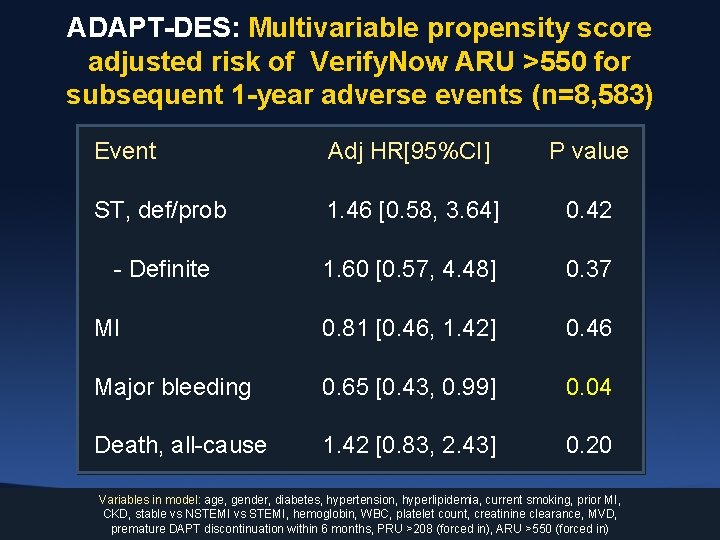
ADAPT-DES: Multivariable propensity score adjusted risk of Verify. Now ARU >550 for subsequent 1 -year adverse events (n=8, 583) Event Adj HR[95%CI] P value ST, def/prob 1. 46 [0. 58, 3. 64] 0. 42 - Definite 1. 60 [0. 57, 4. 48] 0. 37 MI 0. 81 [0. 46, 1. 42] 0. 46 Major bleeding 0. 65 [0. 43, 0. 99] 0. 04 Death, all-cause 1. 42 [0. 83, 2. 43] 0. 20 Variables in model: age, gender, diabetes, hypertension, hyperlipidemia, current smoking, prior MI, CKD, stable vs NSTEMI vs STEMI, hemoglobin, WBC, platelet count, creatinine clearance, MVD, premature DAPT discontinuation within 6 months, PRU >208 (forced in), ARU >550 (forced in)

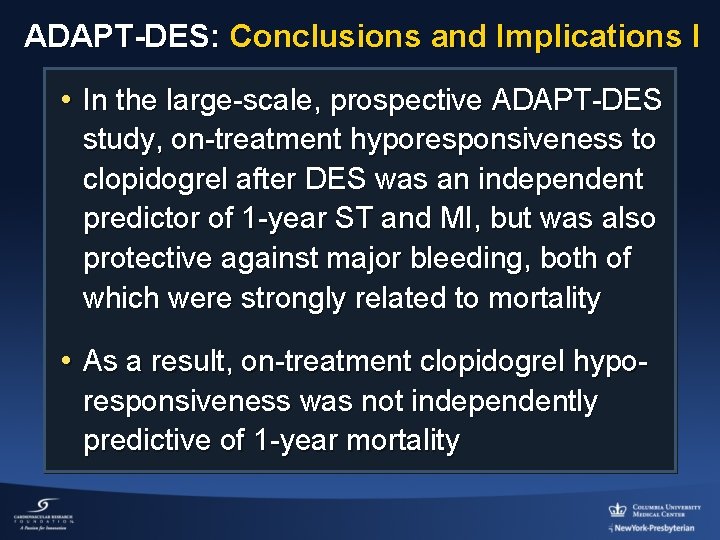
ADAPT-DES: Conclusions and Implications I • In the large-scale, prospective ADAPT-DES study, on-treatment hyporesponsiveness to clopidogrel after DES was an independent predictor of 1 -year ST and MI, but was also protective against major bleeding, both of which were strongly related to mortality • As a result, on-treatment clopidogrel hyporesponsiveness was not independently predictive of 1 -year mortality

ADAPT-DES: Conclusions and Implications II • Overcoming clopidogrel hyporesponsiveness with more potent antiplatelet agents is therefore unlikely to improve survival unless the beneficial effects of reducing ST and MI can be uncoupled from the likely increase in bleeding with greater platelet inhibition • Hyporesponsiveness to aspirin was unrelated to ST, MI or death, but may be related to bleeding, questioning the utility of aspirin in pts treated with DES
 Anti a and anti rh blood type
Anti a and anti rh blood type Dual ii magnum vs dual magnum
Dual ii magnum vs dual magnum Cv range
Cv range Sol gel zone
Sol gel zone Heinz bodies
Heinz bodies Elliptocytosis
Elliptocytosis Fibronogen
Fibronogen Process of platelet plug formation
Process of platelet plug formation Platelet satellitosis
Platelet satellitosis Pbf staining
Pbf staining Indications for platelet transfusion
Indications for platelet transfusion Peripheral blood smear platelet count
Peripheral blood smear platelet count Indications for platelet transfusion
Indications for platelet transfusion Cryoprecipitate
Cryoprecipitate Cbc platelet count low
Cbc platelet count low Brecher cronkite
Brecher cronkite Ffp vs platelets
Ffp vs platelets Lines of zahn is seen in coralline thrombus
Lines of zahn is seen in coralline thrombus How to lower rbc count
How to lower rbc count Platelet aggregation test
Platelet aggregation test In seconds
In seconds Platelet aggregation test
Platelet aggregation test Platelet
Platelet Platelet
Platelet Whats a platelet transfusion
Whats a platelet transfusion