DEScover OneYear Clinical Results Presented at Transcatheter Cardiovascular




- Slides: 14

DEScover: One-Year Clinical Results Presented at Transcatheter Cardiovascular Therapeutics 2006 Presented by Dr. David O. Williams and Dr. J. Dawn Abbott

DEScover One-Year Clinical Results: Study Design • Prospective/observational study • 140 clinical sites in the US • Each site to enroll at least 60 consecutive patients undergoing PCI: – Target -7, 500 patients – Enrollment period: December 2004 –June 2005 • Exclusion criteria: refusal or inability to provide written informed consent and/or HIPAA authorization www. Clinical trial results. org Presented at TCT 2006

DEScover One-Year Clinical Results: Definitions • Death – All cause mortality • Myocardial infarction – Evolutionary ST-segment elevation, or – New Q-waves or LBBB, or – CK>2 ULN and elevated CK-MB or troponin • Stent thrombosis – Acute (0 -24 hours), sub-acute (>24 hours – 30 days), or late (>30 days) – Classified as definite or probable (composite presented) – Adjudicated by an independent events committee • Angiographic characteristics were evaluated at the clinical sites www. Clinical trial results. org Presented at TCT 2006

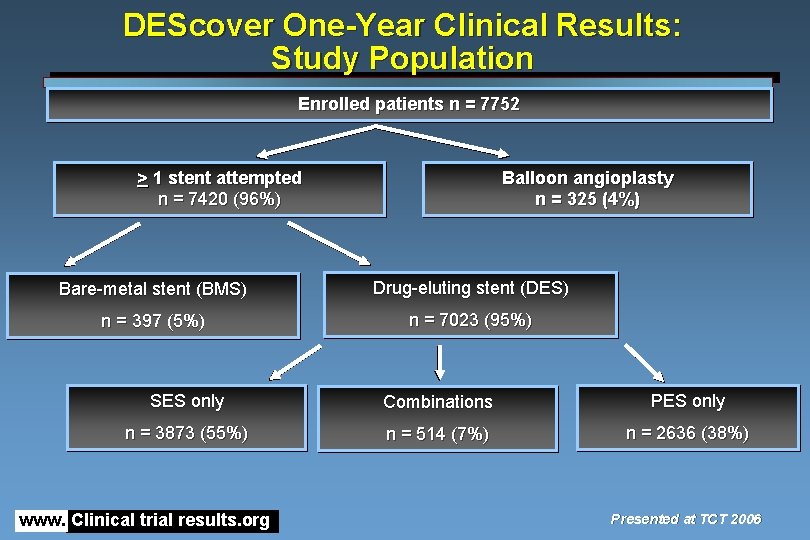
DEScover One-Year Clinical Results: Study Population Enrolled patients n = 7752 > 1 stent attempted n = 7420 (96%) Balloon angioplasty n = 325 (4%) Bare-metal stent (BMS) Drug-eluting stent (DES) n = 397 (5%) n = 7023 (95%) SES only Combinations PES only n = 3873 (55%) n = 514 (7%) n = 2636 (38%) www. Clinical trial results. org Presented at TCT 2006

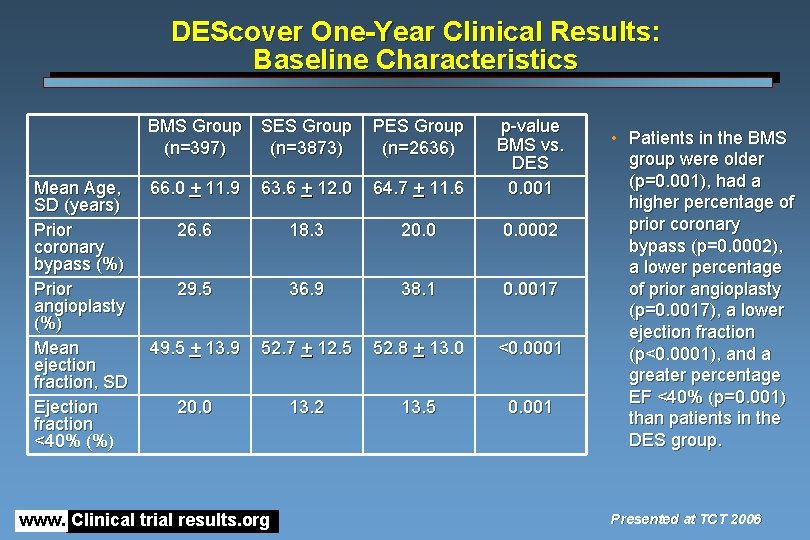
DEScover One-Year Clinical Results: Baseline Characteristics Mean Age, SD (years) Prior coronary bypass (%) Prior angioplasty (%) Mean ejection fraction, SD Ejection fraction <40% (%) BMS Group (n=397) SES Group (n=3873) PES Group (n=2636) 66. 0 + 11. 9 63. 6 + 12. 0 64. 7 + 11. 6 p-value BMS vs. DES 0. 001 26. 6 18. 3 20. 0002 29. 5 36. 9 38. 1 0. 0017 49. 5 + 13. 9 52. 7 + 12. 5 52. 8 + 13. 0 <0. 0001 20. 0 13. 2 13. 5 0. 001 www. Clinical trial results. org • Patients in the BMS group were older (p=0. 001), had a higher percentage of prior coronary bypass (p=0. 0002), a lower percentage of prior angioplasty (p=0. 0017), a lower ejection fraction (p<0. 0001), and a greater percentage EF <40% (p=0. 001) than patients in the DES group. Presented at TCT 2006

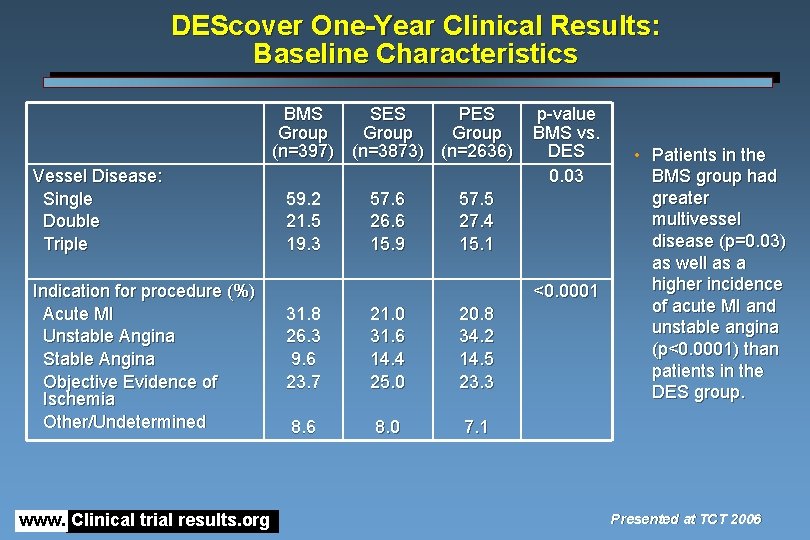
DEScover One-Year Clinical Results: Baseline Characteristics BMS SES PES Group (n=397) (n=3873) (n=2636) Vessel Disease: Single Double Triple Indication for procedure (%) Acute MI Unstable Angina Stable Angina Objective Evidence of Ischemia Other/Undetermined www. Clinical trial results. org 59. 2 21. 5 19. 3 57. 6 26. 6 15. 9 p-value BMS vs. DES 0. 03 57. 5 27. 4 15. 1 <0. 0001 31. 8 26. 3 9. 6 23. 7 21. 0 31. 6 14. 4 25. 0 20. 8 34. 2 14. 5 23. 3 8. 6 8. 0 7. 1 • Patients in the BMS group had greater multivessel disease (p=0. 03) as well as a higher incidence of acute MI and unstable angina (p<0. 0001) than patients in the DES group. Presented at TCT 2006

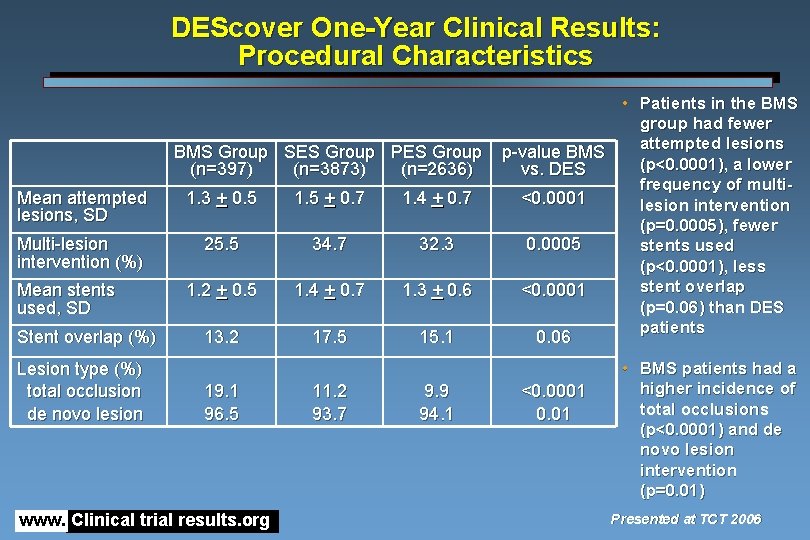
DEScover One-Year Clinical Results: Procedural Characteristics BMS Group SES Group PES Group (n=397) (n=3873) (n=2636) Mean attempted lesions, SD 1. 3 + 0. 5 1. 5 + 0. 7 1. 4 + 0. 7 Multi-lesion intervention (%) 25. 5 34. 7 32. 3 1. 2 + 0. 5 1. 4 + 0. 7 1. 3 + 0. 6 13. 2 17. 5 15. 1 Mean stents used, SD Stent overlap (%) Lesion type (%) total occlusion de novo lesion 19. 1 96. 5 www. Clinical trial results. org 11. 2 93. 7 9. 9 94. 1 • Patients in the BMS group had fewer attempted lesions p-value BMS (p<0. 0001), a lower vs. DES frequency of multi<0. 0001 lesion intervention (p=0. 0005), fewer 0. 0005 stents used (p<0. 0001), less stent overlap <0. 0001 (p=0. 06) than DES patients 0. 06 <0. 0001 0. 01 • BMS patients had a higher incidence of total occlusions (p<0. 0001) and de novo lesion intervention (p=0. 01) Presented at TCT 2006

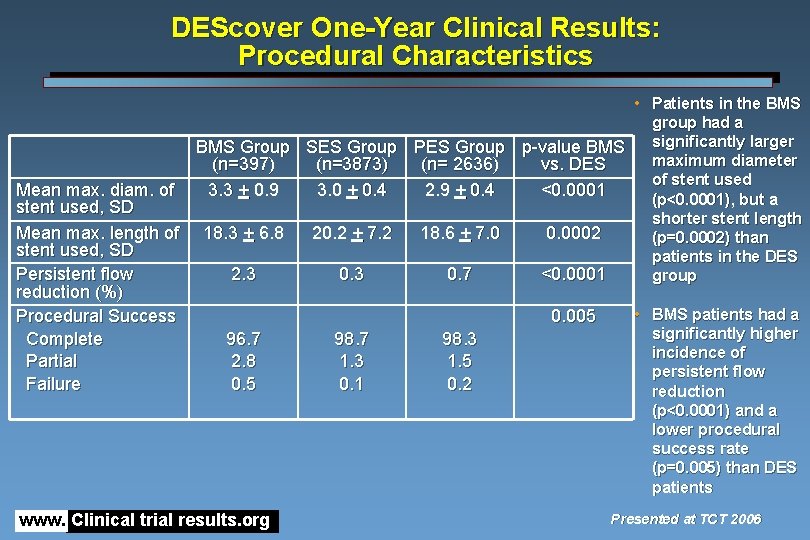
DEScover One-Year Clinical Results: Procedural Characteristics Mean max. diam. of stent used, SD Mean max. length of stent used, SD Persistent flow reduction (%) Procedural Success Complete Partial Failure • Patients in the BMS group had a BMS Group SES Group PES Group p-value BMS significantly larger maximum diameter (n=397) (n=3873) (n= 2636) vs. DES of stent used 3. 3 + 0. 9 3. 0 + 0. 4 2. 9 + 0. 4 <0. 0001 (p<0. 0001), but a shorter stent length 18. 3 + 6. 8 20. 2 + 7. 2 18. 6 + 7. 0 0. 0002 (p=0. 0002) than patients in the DES 2. 3 0. 7 <0. 0001 group 0. 005 96. 7 2. 8 0. 5 www. Clinical trial results. org 98. 7 1. 3 0. 1 98. 3 1. 5 0. 2 • BMS patients had a significantly higher incidence of persistent flow reduction (p<0. 0001) and a lower procedural success rate (p=0. 005) than DES patients Presented at TCT 2006

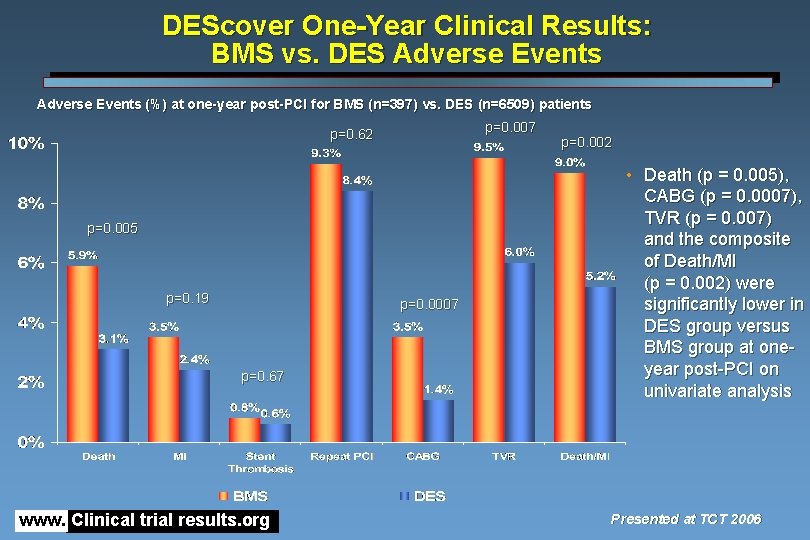
DEScover One-Year Clinical Results: BMS vs. DES Adverse Events (%) at one-year post-PCI for BMS (n=397) vs. DES (n=6509) patients p=0. 007 p=0. 62 p=0. 005 p=0. 19 p=0. 0007 p=0. 67 www. Clinical trial results. org p=0. 002 • Death (p = 0. 005), CABG (p = 0. 0007), TVR (p = 0. 007) and the composite of Death/MI (p = 0. 002) were significantly lower in DES group versus BMS group at oneyear post-PCI on univariate analysis Presented at TCT 2006

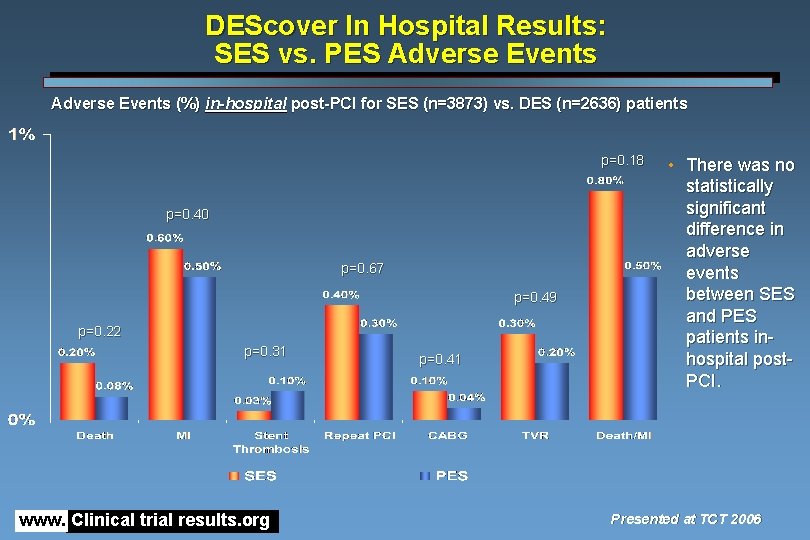
DEScover In Hospital Results: SES vs. PES Adverse Events (%) in-hospital post-PCI for SES (n=3873) vs. DES (n=2636) patients p=0. 18 p=0. 40 p=0. 67 p=0. 49 p=0. 22 p=0. 31 www. Clinical trial results. org p=0. 41 • There was no statistically significant difference in adverse events between SES and PES patients inhospital post. PCI. Presented at TCT 2006

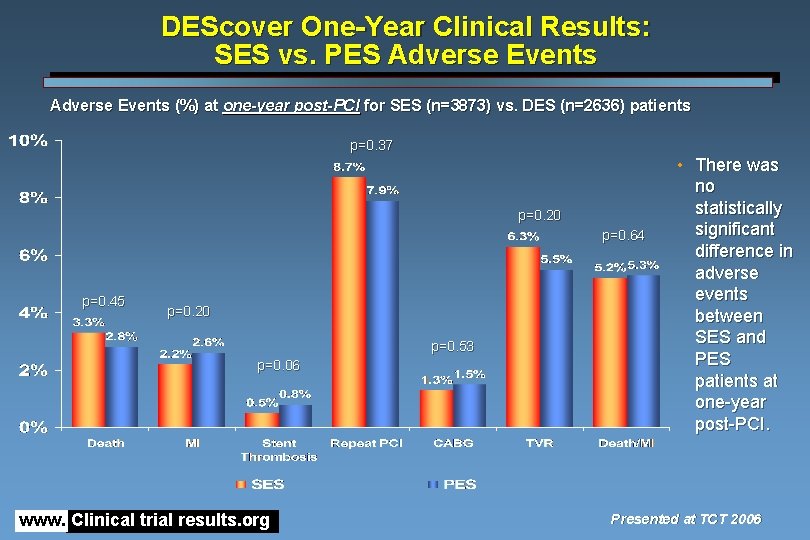
DEScover One-Year Clinical Results: SES vs. PES Adverse Events (%) at one-year post-PCI for SES (n=3873) vs. DES (n=2636) patients p=0. 37 p=0. 20 p=0. 64 p=0. 45 p=0. 20 p=0. 53 p=0. 06 www. Clinical trial results. org • There was no statistically significant difference in adverse events between SES and PES patients at one-year post-PCI. Presented at TCT 2006

DEScover One-Year Clinical Results: BMS vs. DES • Patient selection – BMS placed more often in AMI, CABG – DES preferred for prior stent procedures • Clinical outcomes – Unadjusted data for death favored DES (3. 1% vs. 5. 9%, p=0. 005) and Death/MI (5. 2% vs. 9. 0%, 0. 002) but no difference following adjustment (HR 0. 74, 95% CI 0. 52 -1. 07) – Unadjusted data favored DES for TVR (6. 0% vs. 9. 5%, 0. 007) as well as adjusted results (HR 0. 58, 95% CI 0. 40 -0. 83). www. Clinical trial results. org Presented at TCT 2006

DEScover One-Year Clinical Results: SES vs. PES • Patient selection – Baseline clinical and angiographic features nearly identical • Clinical outcomes – Early, intermediate, and one-year clinical outcomes similar (p=ns) with rates of major adverse events low • Death • MI • Death/MI • Stent thrombosis 3. 3%, 2. 8% 2. 2%, 2. 6% 5. 2%, 5. 3% 0. 5%, 0. 8% – No significant differences in rates of any repeat PCI, CABG, or TVR (6. 3%, 5. 5%) www. Clinical trial results. org Presented at TCT 2006

DEScover One-Year Clinical Results: Limitations • Number of BMS patients relatively small in comparison to DES group • Selection bias between BMS and DES patients (BMS put in STEMI pts, CABG pts etc) • Adjustment may not compensate for baseline differences • No information regarding antiplatelet therapy usage during follow-up • Follow-up beyond one-year desirable www. Clinical trial results. org Presented at TCT 2006
 Descover synonym
Descover synonym Nó sinoatrial
Nó sinoatrial Cardiovascular research institute basel
Cardiovascular research institute basel Pithed rat meaning
Pithed rat meaning Lesson 11 cardiovascular system
Lesson 11 cardiovascular system Paratiroidea
Paratiroidea Crash course circulatory system
Crash course circulatory system Anatomy and physiology unit 7 cardiovascular system
Anatomy and physiology unit 7 cardiovascular system Sistema cardiovascular
Sistema cardiovascular Salud cardiovascular
Salud cardiovascular Soplo protosistolico
Soplo protosistolico Sistolw
Sistolw Tissues in the circulatory system
Tissues in the circulatory system Figure 11-8 arteries
Figure 11-8 arteries Prairie cardiovascular consultants springfield il
Prairie cardiovascular consultants springfield il