Acute HIV Infection New Strategies for Detection Pragna








- Slides: 24

Acute HIV Infection: New Strategies for Detection Pragna Patel MD MPH Centers for Disease Control and Prevention Division of HIV/AIDS Prevention Behavioral and Clinical Surveillance Branch December 5, 2007

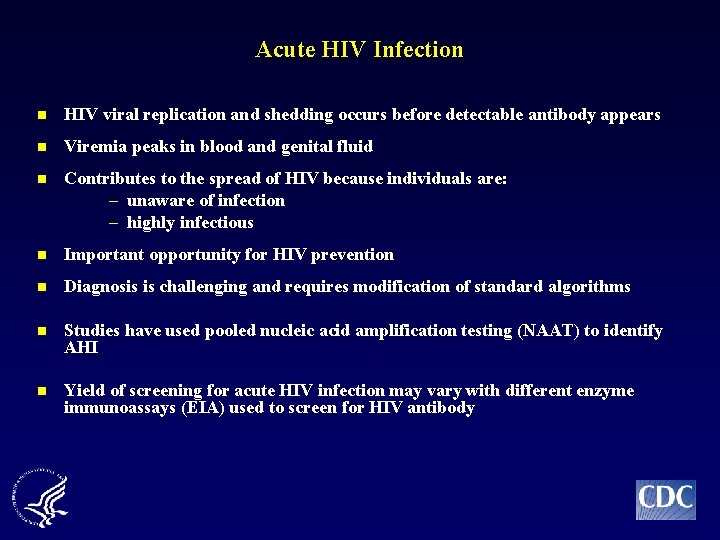
Acute HIV Infection n HIV viral replication and shedding occurs before detectable antibody appears n Viremia peaks in blood and genital fluid n Contributes to the spread of HIV because individuals are: – unaware of infection – highly infectious n Important opportunity for HIV prevention n Diagnosis is challenging and requires modification of standard algorithms n Studies have used pooled nucleic acid amplification testing (NAAT) to identify AHI n Yield of screening for acute HIV infection may vary with different enzyme immunoassays (EIA) used to screen for HIV antibody

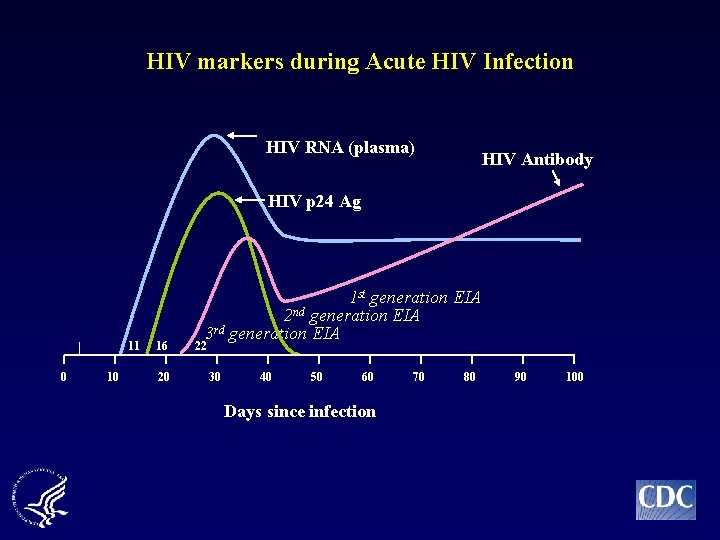
HIV markers during Acute HIV Infection HIV RNA (plasma) HIV Antibody HIV p 24 Ag 11 0 10 16 20 1 st generation EIA 2 nd generation EIA 3 rd generation EIA 22 30 40 50 60 Days since infection 70 80 90 100

AHI Study: Primary Objectives n Assess feasibility and cost-effectiveness of pooled NAAT* relative to 3 rd generation HIV antibody screening assays and rapid tests n Evaluate effect of pool size on sensitivity and cost-effectiveness of pooled NAAT n Identify HIV infections that would otherwise be missed by current testing n Calculate NAAT sensitivity and specificity *NAAT = nucleic acid amplification testing for HIV-1 RNA

AHI Study: Secondary Objectives n Describe AHI epidemiology & transmission risks n Describe antiretroviral resistance in persons with AHI n Estimate number of transmissions averted through diagnosis & subsequent risk reduction n Describe outcomes of partner notification of persons with AHI n Identify AHI transmission clusters using molecular testing and phylogenetic analyses n Estimate entry into care and treatment outcomes

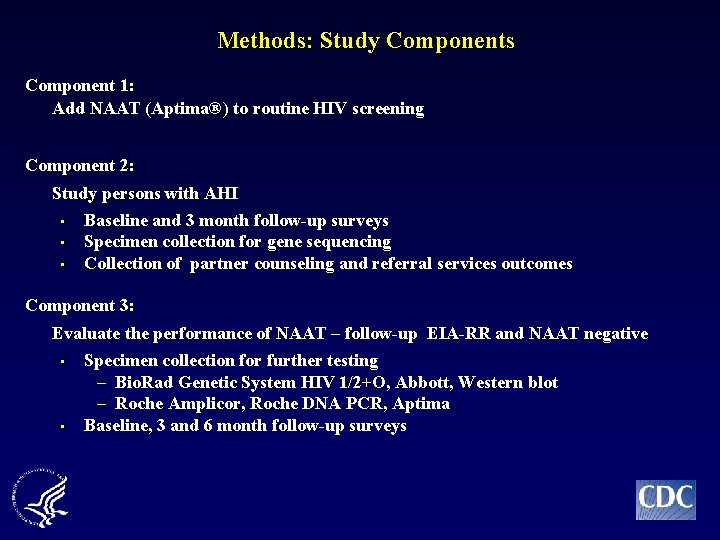
Methods: Study Components Component 1: Add NAAT (Aptima®) to routine HIV screening Component 2: Study persons with AHI • Baseline and 3 month follow-up surveys • Specimen collection for gene sequencing • Collection of partner counseling and referral services outcomes Component 3: Evaluate the performance of NAAT – follow-up EIA-RR and NAAT negative • Specimen collection for further testing – Bio. Rad Genetic System HIV 1/2+O, Abbott, Western blot – Roche Amplicor, Roche DNA PCR, Aptima • Baseline, 3 and 6 month follow-up surveys

Methods: Project Areas § Los Angeles Department of Health Services New York State Department of Health Wadsworth Center, Diagnostic HIV Laboratory § § 14 sexually transmitted disease clinics and one community center Vironostika HIV-1 Microelisa System (less sensitive EIA) Florida Department of Health § § § Florida Bureau of Laboratories 80 public health clinics in 4 counties – Duval, Orange, Hillsborough, Pinellas Bio. Rad Genetic Systems HIV-1/2 plus O (more sensitive EIA) New York City Department of Health and Mental Hygiene New York State Department of Health Wadsworth Center, Diagnostic HIV Laboratory § § 3 sexually transmitted disease clinics – Fort Greene, Morrisania, Chelsea Oraquick Advance Rapid HIV 1/2 (oral fluid) implemented separate consent/counseling for AHI screening

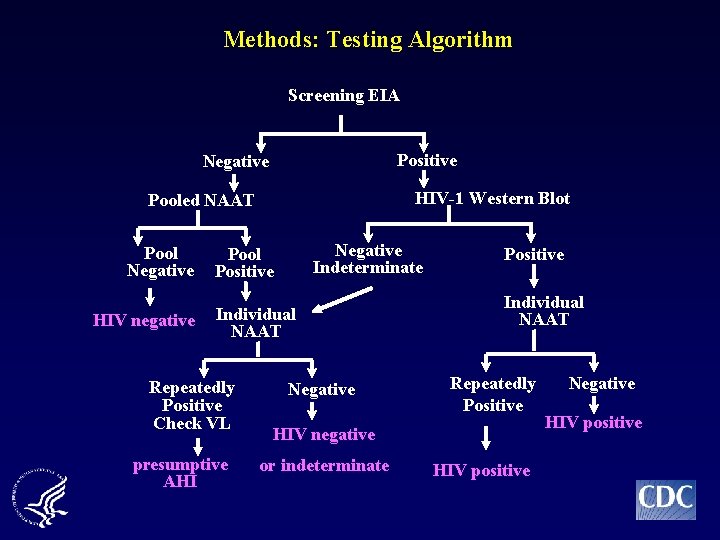
Methods: Testing Algorithm Screening EIA Positive Negative HIV-1 Western Blot Pooled NAAT Pool Negative HIV negative Negative Indeterminate Pool Positive Individual NAAT Repeatedly Positive Check VL presumptive AHI Negative Positive Individual NAAT Repeatedly Positive HIV negative or indeterminate HIV positive Negative HIV positive

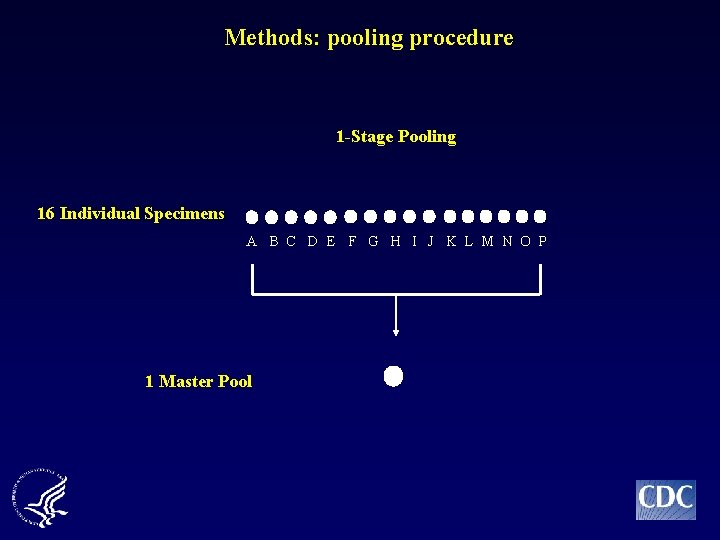
Methods: pooling procedure 1 -Stage Pooling 16 Individual Specimens A B C D E F G H I J K L M N O P 1 Master Pool

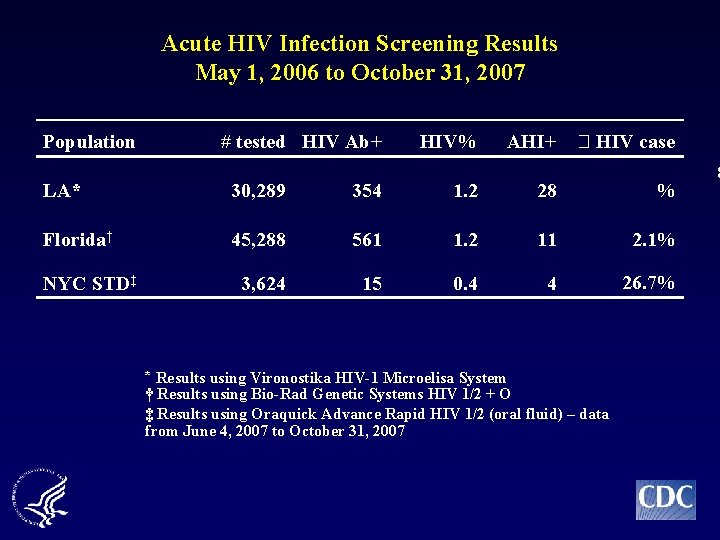
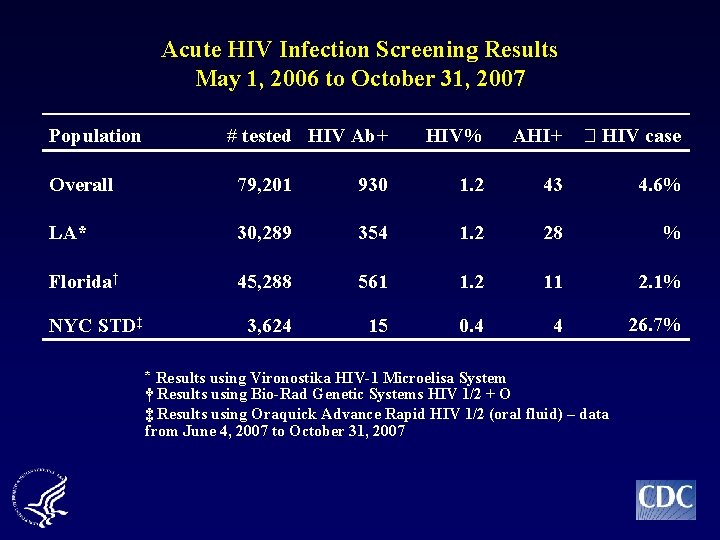
Acute HIV Infection Screening Results May 1, 2006 to October 31, 2007 Population # tested HIV Ab+ HIV% AHI+ HIV case LA* 30, 289 354 1. 2 28 % Florida† 45, 288 561 1. 2 11 2. 1% 3, 624 15 0. 4 4 26. 7% NYC STD‡ * Results using Vironostika HIV-1 Microelisa System † Results using Bio-Rad Genetic Systems HIV 1/2 + O ‡ Results using Oraquick Advance Rapid HIV 1/2 (oral fluid) – data from June 4, 2007 to October 31, 2007 8

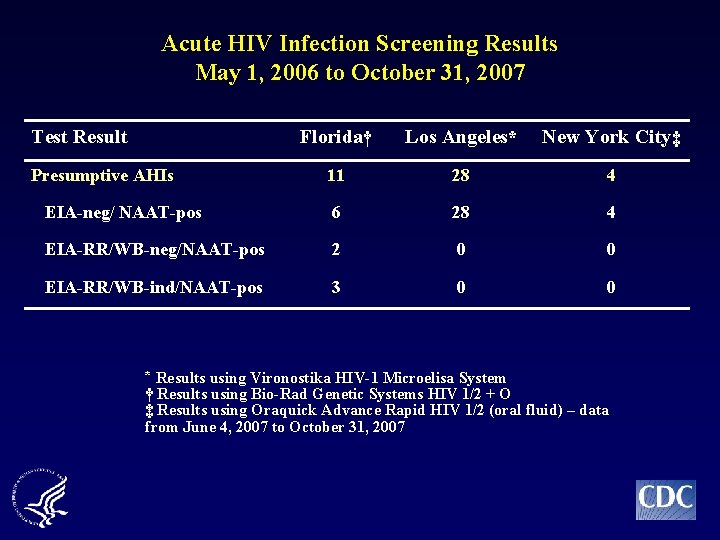
Acute HIV Infection Screening Results May 1, 2006 to October 31, 2007 Test Result Florida† Los Angeles* New York City‡ 11 28 4 EIA-neg/ NAAT-pos 6 28 4 EIA-RR/WB-neg/NAAT-pos 2 0 0 EIA-RR/WB-ind/NAAT-pos 3 0 0 Presumptive AHIs * Results using Vironostika HIV-1 Microelisa System † Results using Bio-Rad Genetic Systems HIV 1/2 + O ‡ Results using Oraquick Advance Rapid HIV 1/2 (oral fluid) – data from June 4, 2007 to October 31, 2007

HIV case detection in LA and NYC using Bio. Rad Genetic Systems 1/2 + O n Tested all acute specimens from LA and NYC using Bio. Rad Genetic Systems 1/2 + O n Los Angeles (n=28) • 12 detected and 16 undetected • Yield of AHI screening would decrease from 8. 0% to 4. 4% n New York City (n=4) • 3 detected and 1 undetected • Yield of AHI screening would decrease from 26. 7% to 5. 5% n Yield of AHI screening higher in LA and NYC compared to FL (2. 1%)

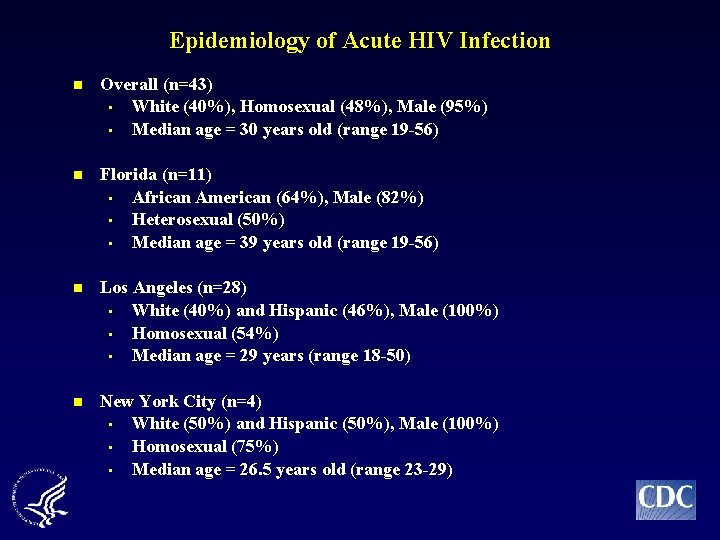
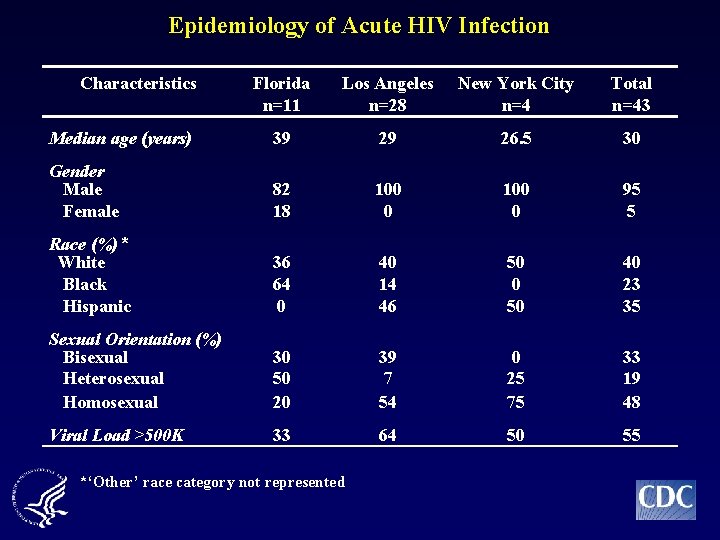
Epidemiology of Acute HIV Infection n Overall (n=43) • White (40%), Homosexual (48%), Male (95%) • Median age = 30 years old (range 19 -56) n Florida (n=11) • African American (64%), Male (82%) • Heterosexual (50%) • Median age = 39 years old (range 19 -56) n Los Angeles (n=28) • White (40%) and Hispanic (46%), Male (100%) • Homosexual (54%) • Median age = 29 years (range 18 -50) n New York City (n=4) • White (50%) and Hispanic (50%), Male (100%) • Homosexual (75%) • Median age = 26. 5 years old (range 23 -29)

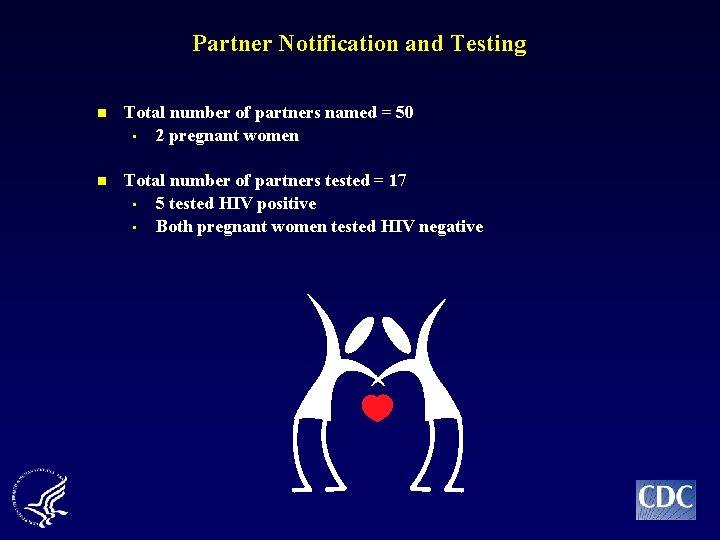
Partner Notification and Testing n Total number of partners named = 50 • 2 pregnant women n Total number of partners tested = 17 • 5 tested HIV positive • Both pregnant women tested HIV negative

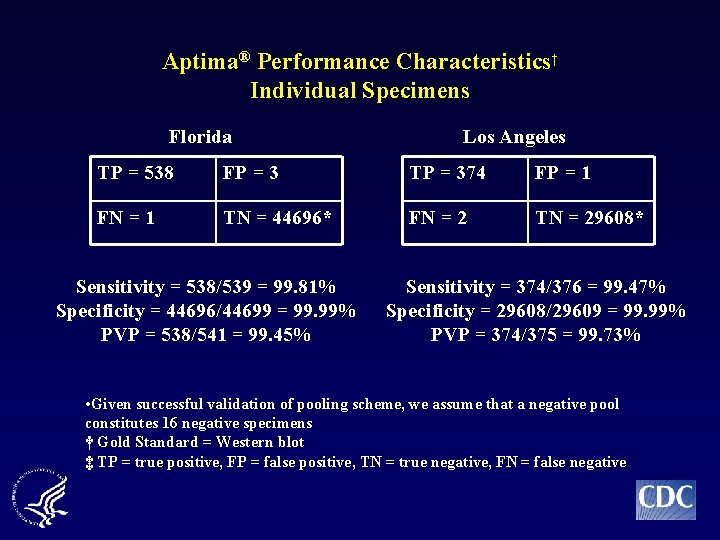
Aptima® Performance Characteristics† Individual Specimens Florida Los Angeles TP = 538 FP = 3 TP = 374 FP = 1 FN = 1 TN = 44696* FN = 2 TN = 29608* Sensitivity = 538/539 = 99. 81% Specificity = 44696/44699 = 99. 99% PVP = 538/541 = 99. 45% Sensitivity = 374/376 = 99. 47% Specificity = 29608/29609 = 99. 99% PVP = 374/375 = 99. 73% • Given successful validation of pooling scheme, we assume that a negative pool constitutes 16 negative specimens † Gold Standard = Western blot ‡ TP = true positive, FP = false positive, TN = true negative, FN = false negative

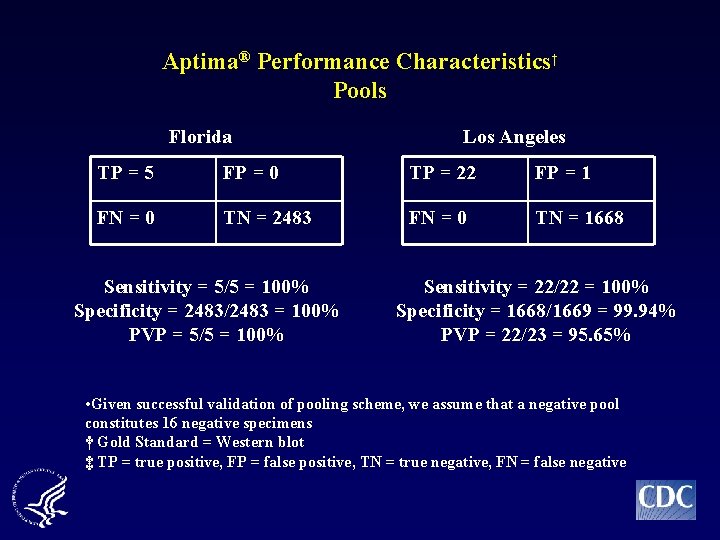
Aptima® Performance Characteristics† Pools Florida Los Angeles TP = 5 FP = 0 TP = 22 FP = 1 FN = 0 TN = 2483 FN = 0 TN = 1668 Sensitivity = 5/5 = 100% Specificity = 2483/2483 = 100% PVP = 5/5 = 100% Sensitivity = 22/22 = 100% Specificity = 1668/1669 = 99. 94% PVP = 22/23 = 95. 65% • Given successful validation of pooling scheme, we assume that a negative pool constitutes 16 negative specimens † Gold Standard = Western blot ‡ TP = true positive, FP = false positive, TN = true negative, FN = false negative

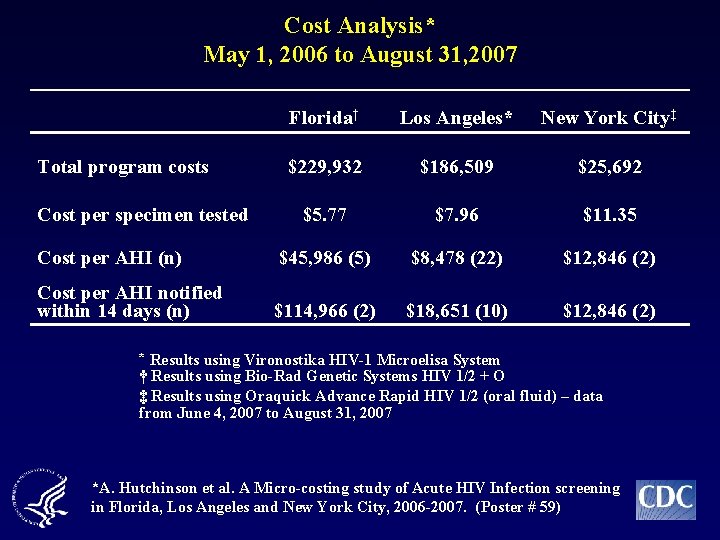
Cost Analysis* May 1, 2006 to August 31, 2007 Florida† Los Angeles* New York City‡ $229, 932 $186, 509 $25, 692 $5. 77 $7. 96 $11. 35 Cost per AHI (n) $45, 986 (5) $8, 478 (22) $12, 846 (2) Cost per AHI notified within 14 days (n) $114, 966 (2) $18, 651 (10) $12, 846 (2) Total program costs Cost per specimen tested * Results using Vironostika HIV-1 Microelisa System † Results using Bio-Rad Genetic Systems HIV 1/2 + O ‡ Results using Oraquick Advance Rapid HIV 1/2 (oral fluid) – data from June 4, 2007 to August 31, 2007 *A. Hutchinson et al. A Micro-costing study of Acute HIV Infection screening in Florida, Los Angeles and New York City, 2006 -2007. (Poster # 59)

Conclusions n Pooled NAAT in addition to 3 rd generation and rapid EIA screening increases HIV case detection n Routine pooling will be most successful with timely notification of results n Aptima® performed well on pooled specimens from routine testing populations n AHI screening using pooled NAAT can be implemented at a cost similar to other HIV screening programs

Acknowledgements CDC Duncan Mackellar Patrick Sullivan Steven Ethridge Steven Mc. Dougal Michele Owen Walid Heneine Clyde Hart Angela Hutchinson Stephanie Sansom Paul Farnham NYS Wadsworth Lab Monica Parker Tim Sullivan Kathy Gombel Florida BOL Berry Bennett Florida DOH Marlene La. Lota Pat Simmons Dan George Melinda Waters Dan Pope Robert Chen Stacy Shiver NYC DOHMH Kate Gallagher Alexis Kowalski Steven Rubin Susan Blank Los Angeles DHS STD Peter Kerndt Apurva Uniyal Ali Stirland Michael Chen Stacei Morita La. Shawnda Royal Kai-Jen Chang

Epidemiology of Acute HIV Infection Characteristics Florida n=11 Los Angeles n=28 New York City n=4 Total n=43 Median age (years) 39 29 26. 5 30 Gender Male Female 82 18 100 0 95 5 Race (%) * White Black Hispanic 36 64 0 40 14 46 50 0 50 40 23 35 Sexual Orientation (%) Bisexual Heterosexual Homosexual 30 50 20 39 7 54 0 25 75 33 19 48 Viral Load >500 K 33 64 50 55 *‘Other’ race category not represented

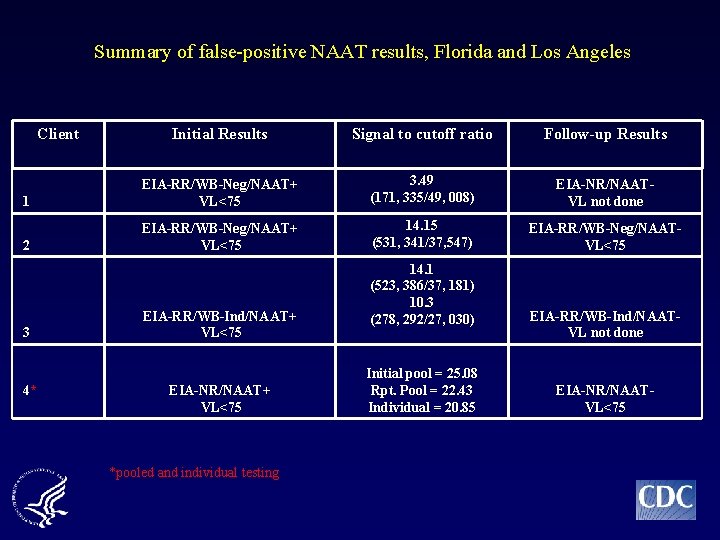
Summary of false-positive NAAT results, Florida and Los Angeles Client Initial Results Signal to cutoff ratio Follow-up Results 1 EIA-RR/WB-Neg/NAAT+ VL<75 3. 49 (171, 335/49, 008) EIA-NR/NAATVL not done 2 EIA-RR/WB-Neg/NAAT+ VL<75 14. 15 (531, 341/37, 547) EIA-RR/WB-Neg/NAATVL<75 3 4* EIA-RR/WB-Ind/NAAT+ VL<75 EIA-NR/NAAT+ VL<75 *pooled and individual testing 14. 1 (523, 386/37, 181) 10. 3 (278, 292/27, 030) Initial pool = 25. 08 Rpt. Pool = 22. 43 Individual = 20. 85 EIA-RR/WB-Ind/NAATVL not done EIA-NR/NAATVL<75

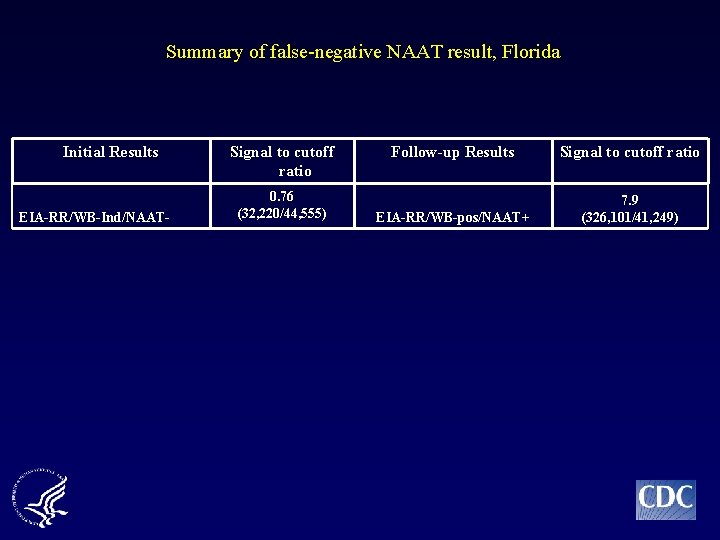
Summary of false-negative NAAT result, Florida Initial Results EIA-RR/WB-Ind/NAAT- Signal to cutoff ratio 0. 76 (32, 220/44, 555) Follow-up Results Signal to cutoff ratio EIA-RR/WB-pos/NAAT+ 7. 9 (326, 101/41, 249)

Acute HIV Infection Screening Results May 1, 2006 to October 31, 2007 Test Result Florida* Los Angeles† New York City‡ Total # tested 45, 288 30, 289 3, 624 EIA-neg/NAAT-neg 44, 638 29, 589 3, 597 EIA-RR/WB-pos/NAAT-pos 528 352 15 Presumptive AHIs 11 28 4 EIA-neg/ NAAT-pos 6 28 4 EIA-RR/WB-neg/NAAT-pos 2 0 0 EIA-RR/WB-ind/NAAT-pos 3 0 0 EIA-RR/WB-neg/NAAT-neg 58 19 3 EIA-RR/WB-ind/NAAT-neg 16 9 0 EIA-RR/WB-pos/NAAT-neg 33 2 0 * Results using Bio. Rad HIV-1/2 plus O † Results using Vironostika HIV-1 Microelisa System ‡ Results using Oraquick Advance Rapid HIV 1/2 (oral fluid) – data from June 4, 2007 to October 31, 2007

Acute HIV Infection Screening Results May 1, 2006 to October 31, 2007 Population # tested HIV Ab+ HIV% AHI+ HIV case Overall 79, 201 930 1. 2 43 4. 6% LA* 30, 289 354 1. 2 28 % Florida† 45, 288 561 1. 2 11 2. 1% 3, 624 15 0. 4 4 26. 7% NYC STD‡ * Results using Vironostika HIV-1 Microelisa System † Results using Bio-Rad Genetic Systems HIV 1/2 + O ‡ Results using Oraquick Advance Rapid HIV 1/2 (oral fluid) – data from June 4, 2007 to October 31, 2007
 Acute specific surgical infection
Acute specific surgical infection Protective reflexes
Protective reflexes Active phagocytes that increase rapidly acute infection
Active phagocytes that increase rapidly acute infection Kontinuitetshantering i praktiken
Kontinuitetshantering i praktiken Typiska novell drag
Typiska novell drag Nationell inriktning för artificiell intelligens
Nationell inriktning för artificiell intelligens Vad står k.r.å.k.a.n för
Vad står k.r.å.k.a.n för Shingelfrisyren
Shingelfrisyren En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Personalliggare bygg undantag
Personalliggare bygg undantag Tidbok för yrkesförare
Tidbok för yrkesförare Sura för anatom
Sura för anatom Vad är densitet
Vad är densitet Datorkunskap för nybörjare
Datorkunskap för nybörjare Stig kerman
Stig kerman Debatt mall
Debatt mall Autokratiskt ledarskap
Autokratiskt ledarskap Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Påbyggnader för flakfordon
Påbyggnader för flakfordon Tryck formel
Tryck formel Svenskt ramverk för digital samverkan
Svenskt ramverk för digital samverkan Jag har nigit för nymånens skära text
Jag har nigit för nymånens skära text Presentera för publik crossboss
Presentera för publik crossboss Vad är ett minoritetsspråk
Vad är ett minoritetsspråk Bat mitza
Bat mitza