8 7 Nucleophiles and Nucleophilicity Nucleophiles The nucleophiles






- Slides: 19

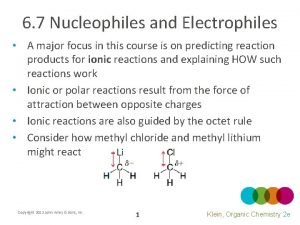
8. 7 Nucleophiles and Nucleophilicity

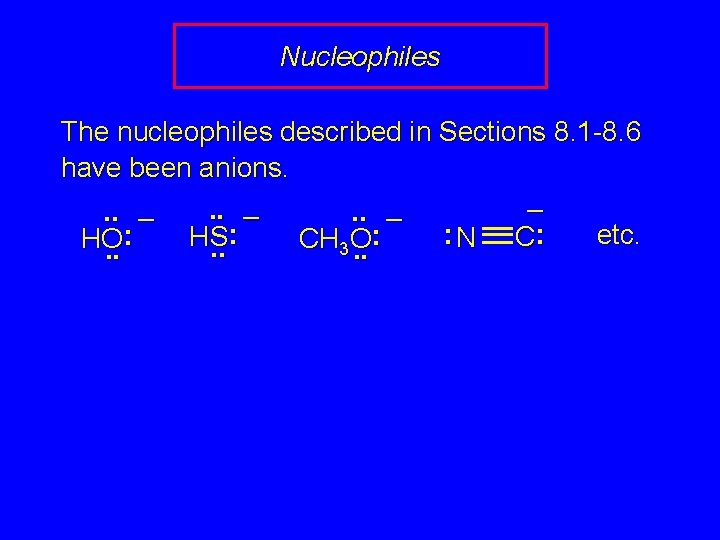
Nucleophiles The nucleophiles described in Sections 8. 1 -8. 6 have been anions. –. . – : etc. : : HS C N HO CH O 3. . .

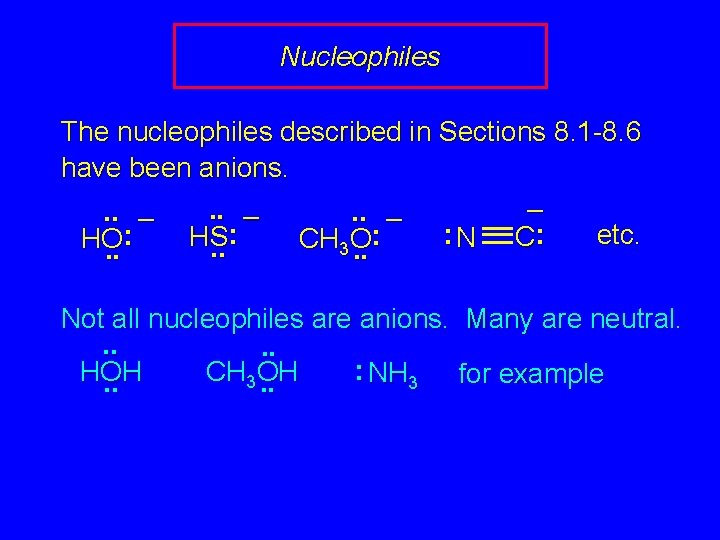
Nucleophiles The nucleophiles described in Sections 8. 1 -8. 6 have been anions. –. . – : etc. : : HS C N HO CH O 3. . . Not all nucleophiles are anions. Many are neutral. . . : CH OH HOH NH 3 for example 3. .

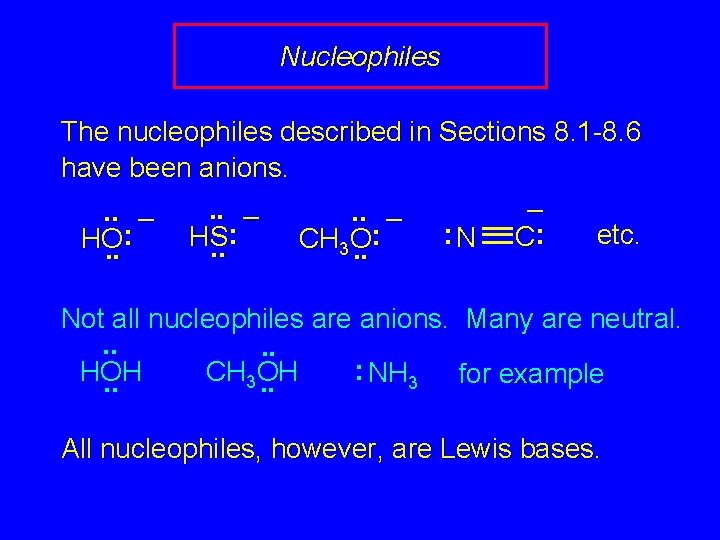
Nucleophiles The nucleophiles described in Sections 8. 1 -8. 6 have been anions. –. . – : etc. : : HS C N HO CH O 3. . . Not all nucleophiles are anions. Many are neutral. . . : CH OH HOH NH 3 for example 3. . All nucleophiles, however, are Lewis bases.

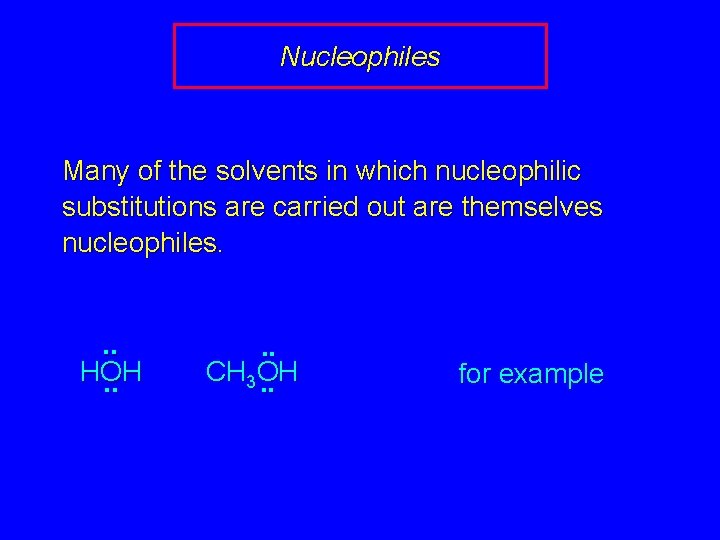
Nucleophiles Many of the solvents in which nucleophilic substitutions are carried out are themselves nucleophiles. . . HOH. . CH 3 OH. . for example

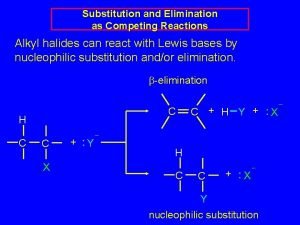
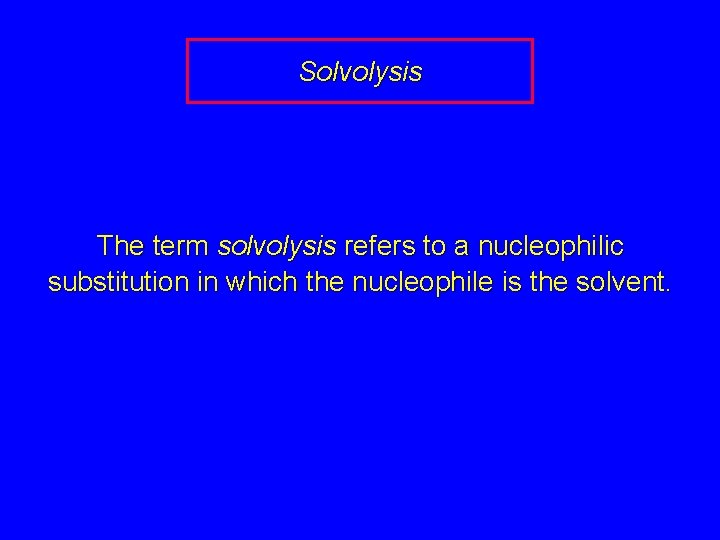
Solvolysis The term solvolysis refers to a nucleophilic substitution in which the nucleophile is the solvent.

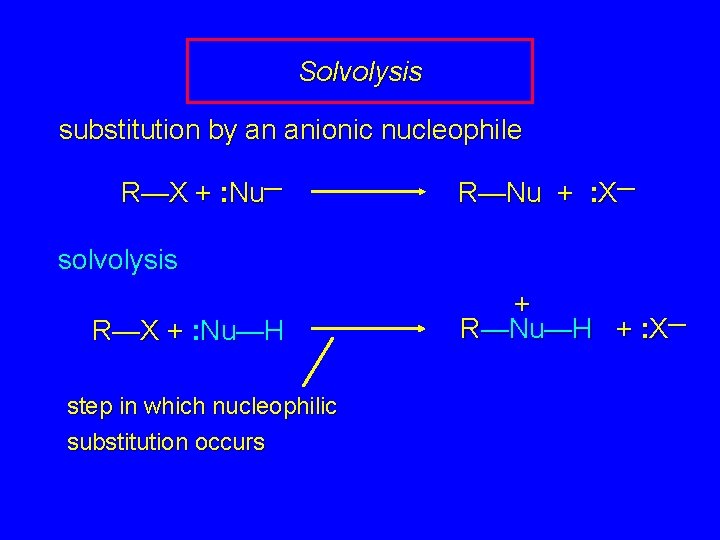
Solvolysis substitution by an anionic nucleophile R—X + : Nu— R—Nu + : X— solvolysis R—X + : Nu—H step in which nucleophilic substitution occurs + R—Nu—H + : X—

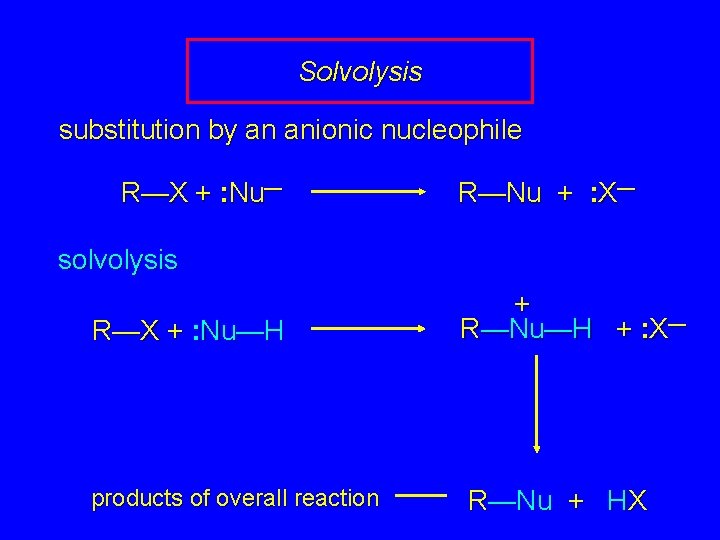
Solvolysis substitution by an anionic nucleophile R—X + : Nu— R—Nu + : X— solvolysis R—X + : Nu—H products of overall reaction + R—Nu—H + : X— R—Nu + HX

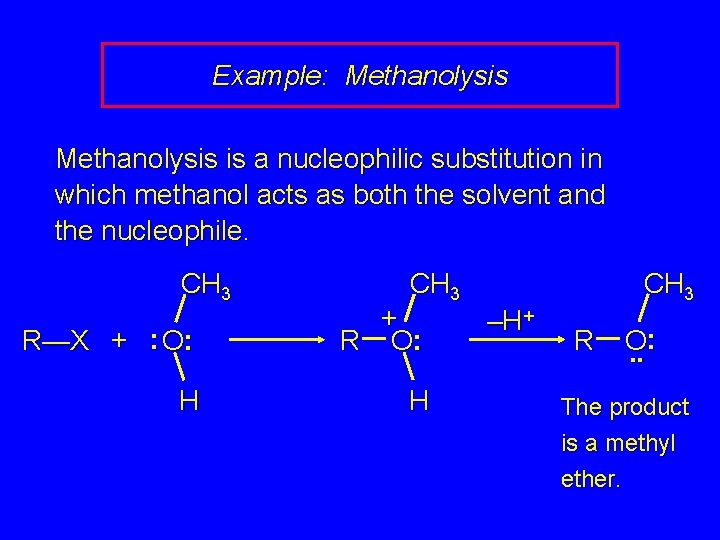
Example: Methanolysis is a nucleophilic substitution in which methanol acts as both the solvent and the nucleophile. CH 3 R—X + : O: + R O: H H –H+ R O. . : The product is a methyl ether.

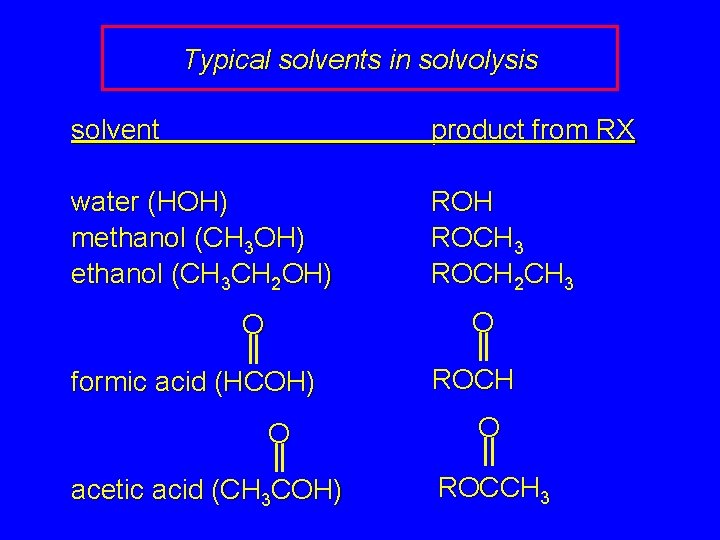
Typical solvents in solvolysis solvent product from RX water (HOH) methanol (CH 3 OH) ethanol (CH 3 CH 2 OH) ROH ROCH 3 ROCH 2 CH 3 O O formic acid (HCOH) O acetic acid (CH 3 COH) ROCH O ROCCH 3

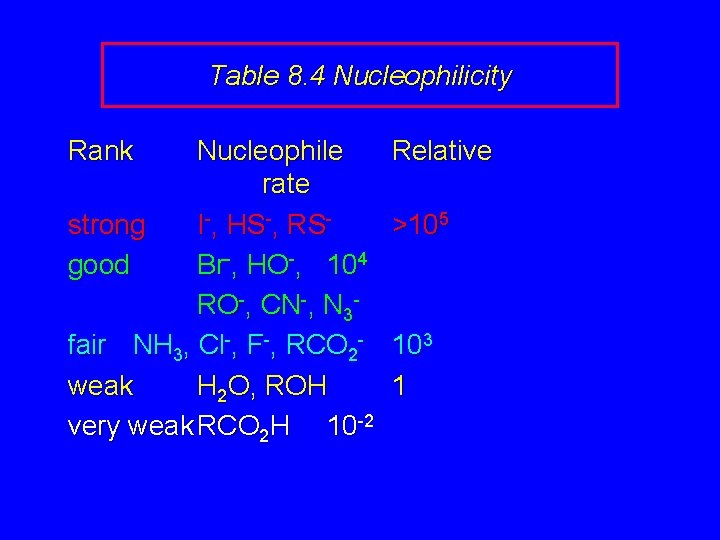
Nucleophilicity is a measure of the reactivity of a nucleophile Table 8. 4 compares the relative rates of nucleophilic substitution of a variety of nucleophiles toward methyl iodide as the substrate. The standard of comparison is methanol, which is assigned a relative rate of 1. 0.

Table 8. 4 Nucleophilicity Rank Nucleophile rate strong I-, HS-, RSgood Br-, HO-, 104 RO-, CN-, N 3 fair NH 3, Cl-, F-, RCO 2 weak H 2 O, ROH very weak RCO 2 H 10 -2 Relative >105 103 1

Major factors that control nucleophilicity basicity solvation small negative ions are highly solvated in protic solvents large negative ions are less solvated polarizability

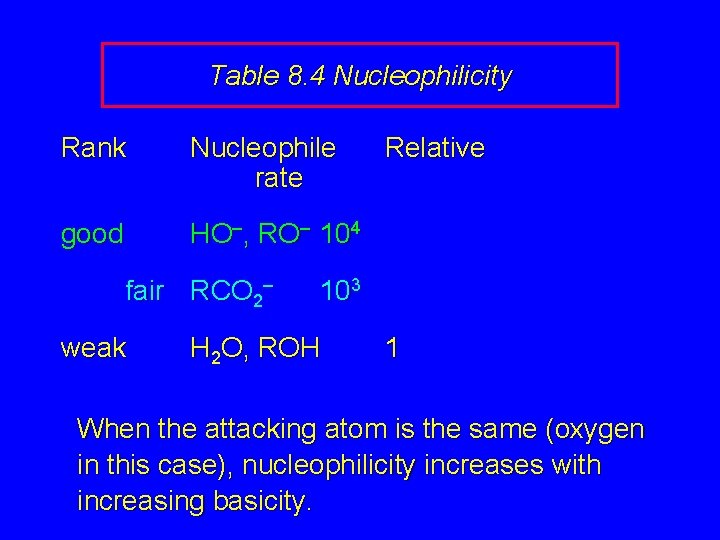
Table 8. 4 Nucleophilicity Rank Nucleophile rate good HO–, RO– 104 fair RCO 2– weak Relative 103 H 2 O, ROH 1 When the attacking atom is the same (oxygen in this case), nucleophilicity increases with increasing basicity.

Major factors that control nucleophilicity basicity solvation small negative ions are highly solvated in protic solvents large negative ions are less solvated polarizability

Figure 8. 4 Solvation of a chloride ion by ion-dipole attractive forces with water. The negatively charged chloride ion interacts with the positively polarized hydrogens of water.

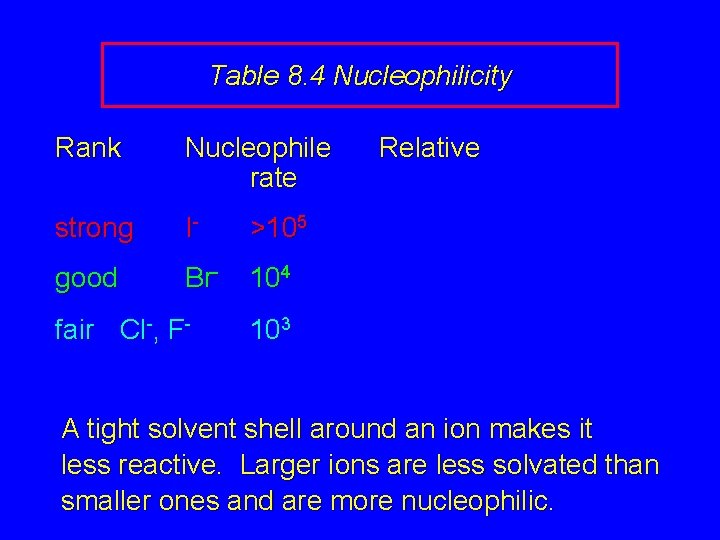
Table 8. 4 Nucleophilicity Rank Nucleophile rate strong I- >105 good Br- 104 fair Cl-, F- Relative 103 A tight solvent shell around an ion makes it less reactive. Larger ions are less solvated than smaller ones and are more nucleophilic.

Major factors that control nucleophilicity basicity solvation small negative ions are highly solvated in protic solvents large negative ions are less solvated polarizability

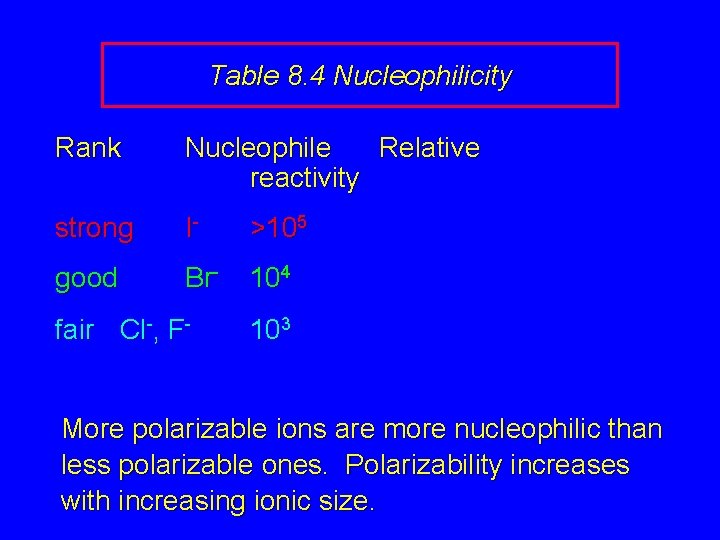
Table 8. 4 Nucleophilicity Rank Nucleophile Relative reactivity strong I- >105 good Br- 104 fair Cl-, F- 103 More polarizable ions are more nucleophilic than less polarizable ones. Polarizability increases with increasing ionic size.
 Nucleophilicity trend
Nucleophilicity trend Alkyl halide
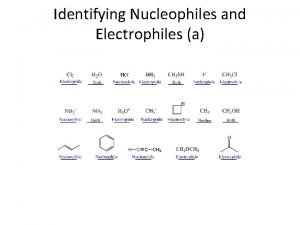
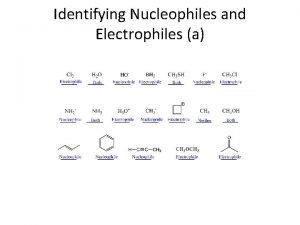
Alkyl halide Identifying electrophiles and nucleophiles
Identifying electrophiles and nucleophiles Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Ng-html
Ng-html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Thang điểm glasgow
Thang điểm glasgow Hát lên người ơi alleluia
Hát lên người ơi alleluia Môn thể thao bắt đầu bằng từ đua
Môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Phép trừ bù
Phép trừ bù Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống