Identifying Nucleophiles and Electrophiles a Identifying Nucleophiles and
- Slides: 12

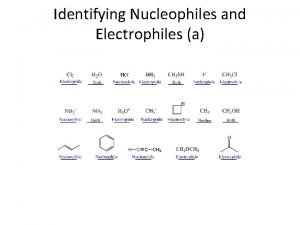
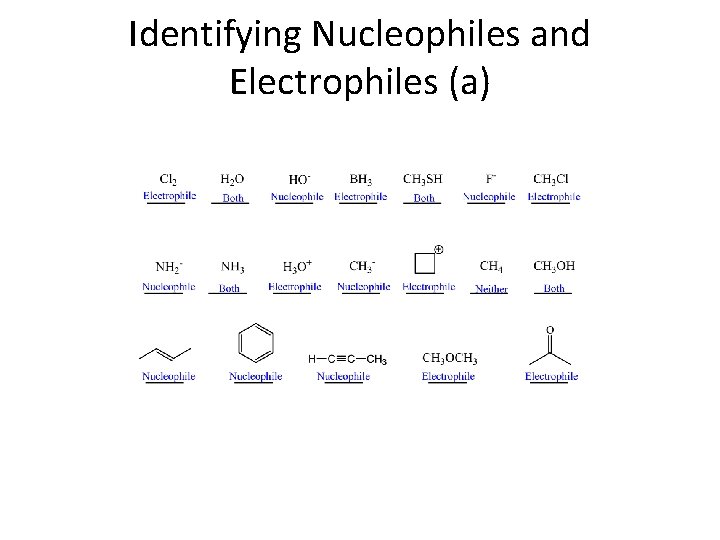
Identifying Nucleophiles and Electrophiles (a)

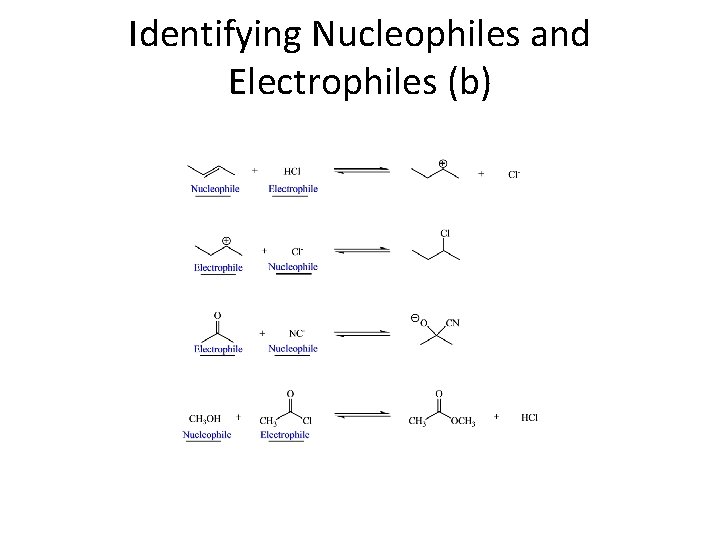
Identifying Nucleophiles and Electrophiles (b)

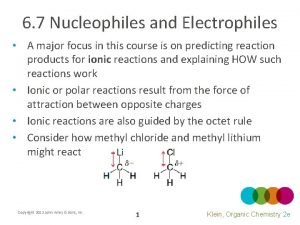
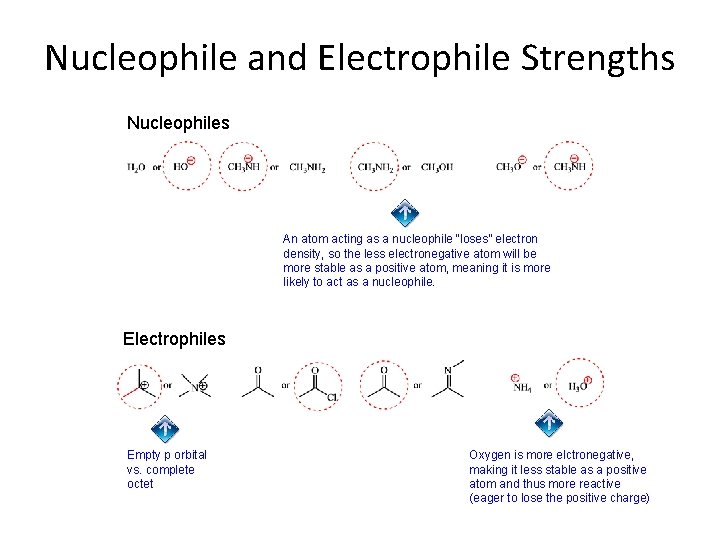
Nucleophile and Electrophile Strengths Nucleophiles An atom acting as a nucleophile “loses” electron density, so the less electronegative atom will be more stable as a positive atom, meaning it is more likely to act as a nucleophile. Electrophiles Empty p orbital vs. complete octet Oxygen is more elctronegative, making it less stable as a positive atom and thus more reactive (eager to lose the positive charge)

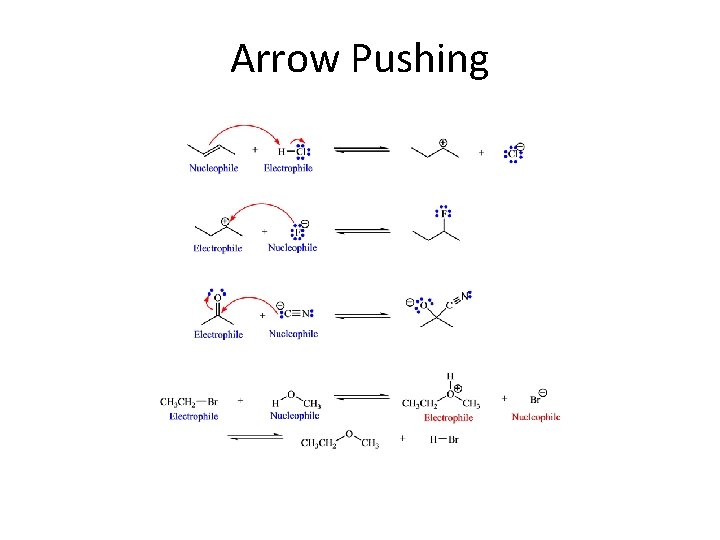
Arrow Pushing

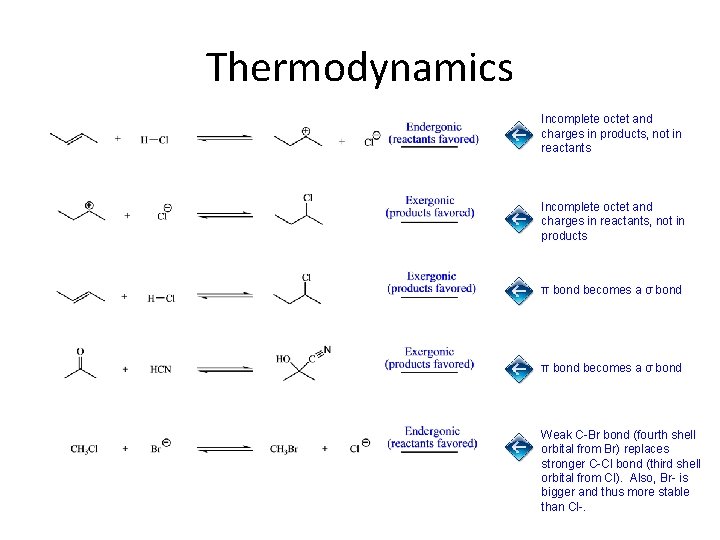
Thermodynamics Incomplete octet and charges in products, not in reactants Incomplete octet and charges in reactants, not in products π bond becomes a σ bond Weak C-Br bond (fourth shell orbital from Br) replaces stronger C-Cl bond (third shell orbital from Cl). Also, Br- is bigger and thus more stable than Cl-.

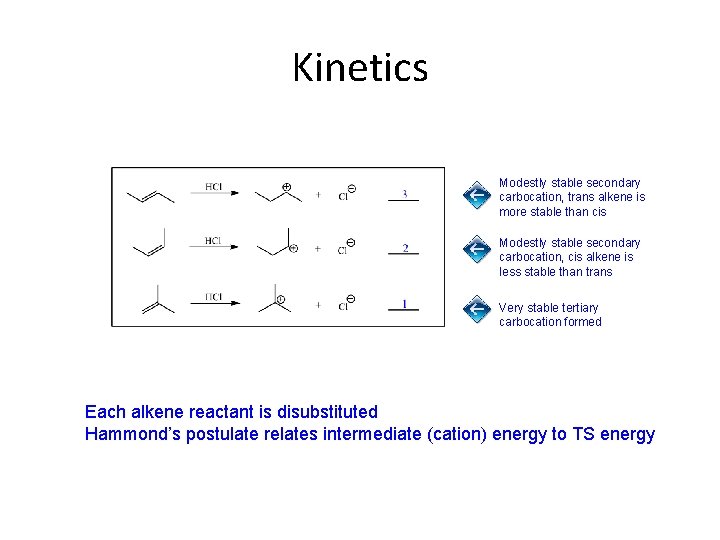
Kinetics Modestly stable secondary carbocation, trans alkene is more stable than cis Modestly stable secondary carbocation, cis alkene is less stable than trans Very stable tertiary carbocation formed Each alkene reactant is disubstituted Hammond’s postulate relates intermediate (cation) energy to TS energy

Free Energy Diagrams (a)

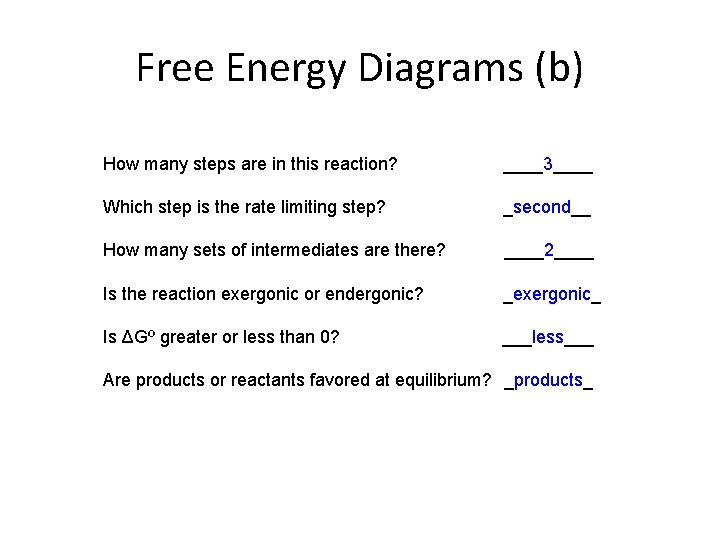
Free Energy Diagrams (b) How many steps are in this reaction? ____3____ Which step is the rate limiting step? _second__ How many sets of intermediates are there? ____2____ Is the reaction exergonic or endergonic? _exergonic_ Is ΔGº greater or less than 0? ___less___ Are products or reactants favored at equilibrium? _products_

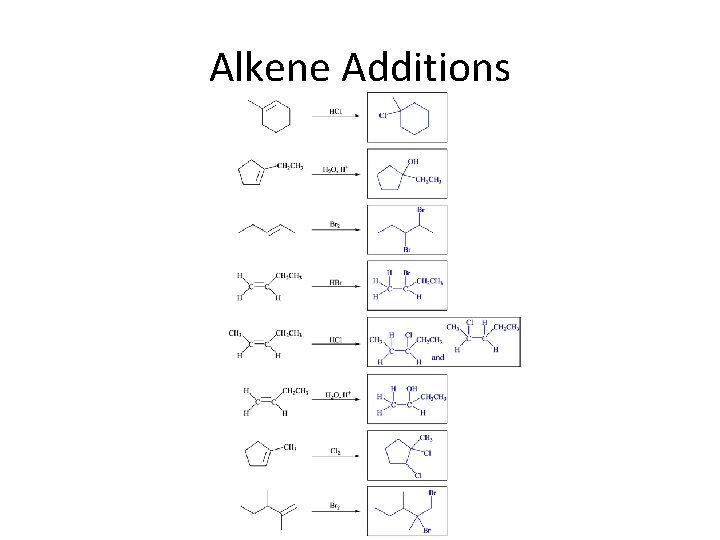
Alkene Additions

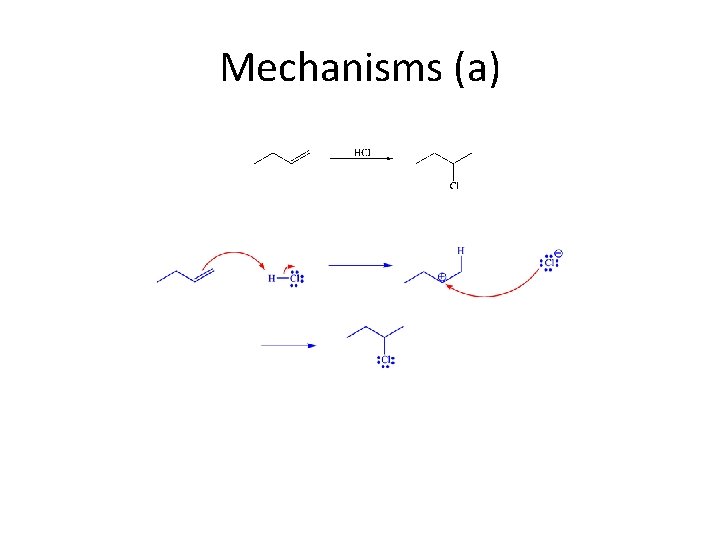
Mechanisms (a)

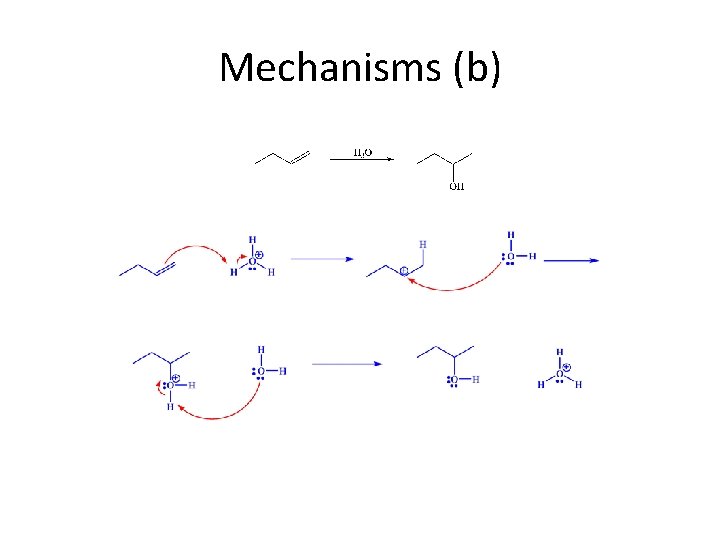
Mechanisms (b)

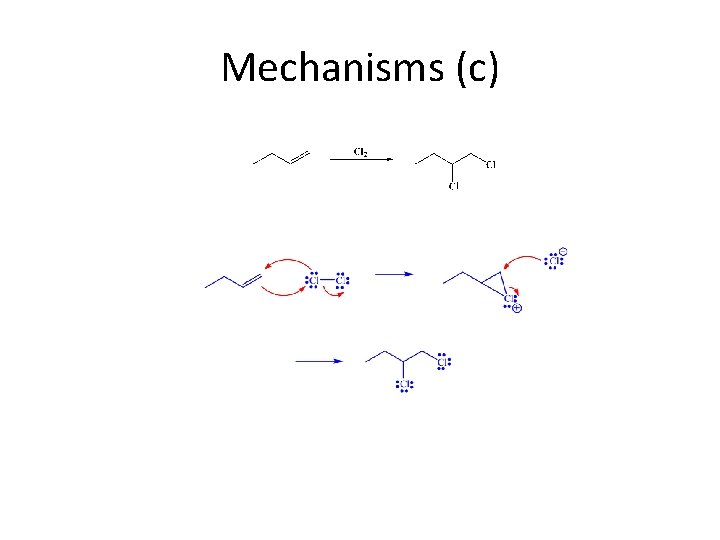
Mechanisms (c)
 Identifying electrophiles and nucleophiles
Identifying electrophiles and nucleophiles Hydrogenation
Hydrogenation Identifying and non identifying adjective clauses
Identifying and non identifying adjective clauses Adjective clause identification
Adjective clause identification Identifying and non identifying adjective clauses
Identifying and non identifying adjective clauses Dependent variable function
Dependent variable function Style and tone examples
Style and tone examples Style vs tone
Style vs tone How to identify the oxidizing agent
How to identify the oxidizing agent Identifying and correcting faulty sentences
Identifying and correcting faulty sentences Mis bidgoli
Mis bidgoli 6-1 identifying and representing functions
6-1 identifying and representing functions Identifying the inquiry and stating the problem
Identifying the inquiry and stating the problem