8 3 The Concentration of Solutions What is







- Slides: 15

8. 3 The Concentration of Solutions

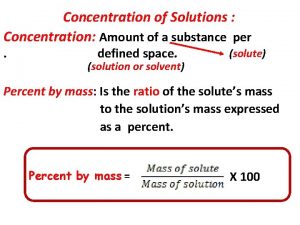
What is Concentration? • Concentration tell us the amount of solute per quantity of solvent.

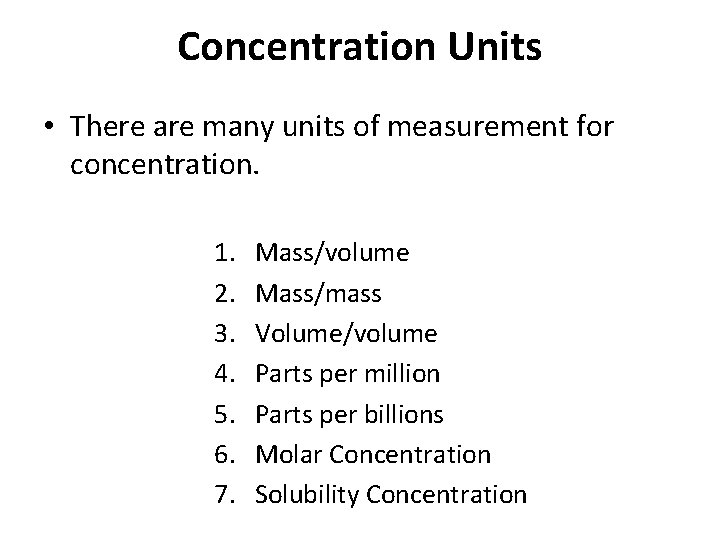
Concentration Units • There are many units of measurement for concentration. 1. 2. 3. 4. 5. 6. 7. Mass/volume Mass/mass Volume/volume Parts per million Parts per billions Molar Concentration Solubility Concentration

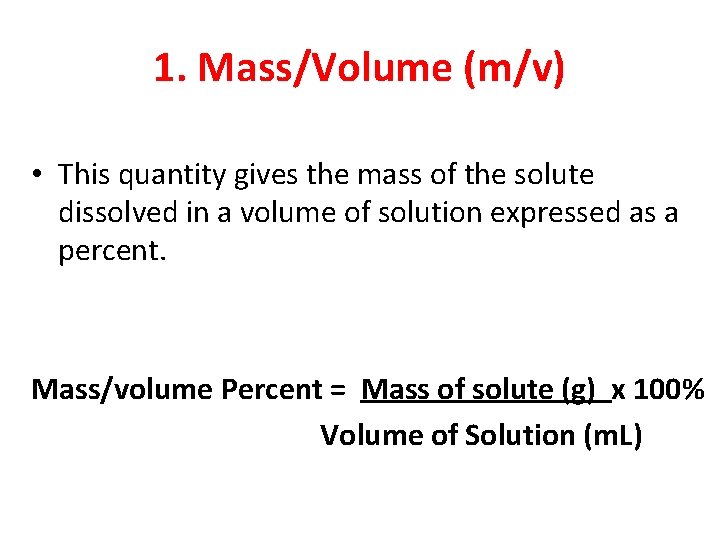
1. Mass/Volume (m/v) • This quantity gives the mass of the solute dissolved in a volume of solution expressed as a percent. Mass/volume Percent = Mass of solute (g) x 100% Volume of Solution (m. L)

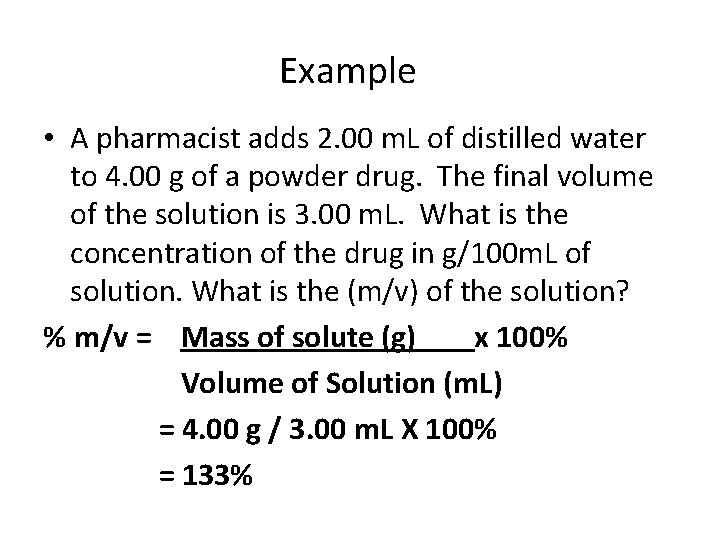
Example • A pharmacist adds 2. 00 m. L of distilled water to 4. 00 g of a powder drug. The final volume of the solution is 3. 00 m. L. What is the concentration of the drug in g/100 m. L of solution. What is the (m/v) of the solution? % m/v = Mass of solute (g) x 100% Volume of Solution (m. L) = 4. 00 g / 3. 00 m. L X 100% = 133%

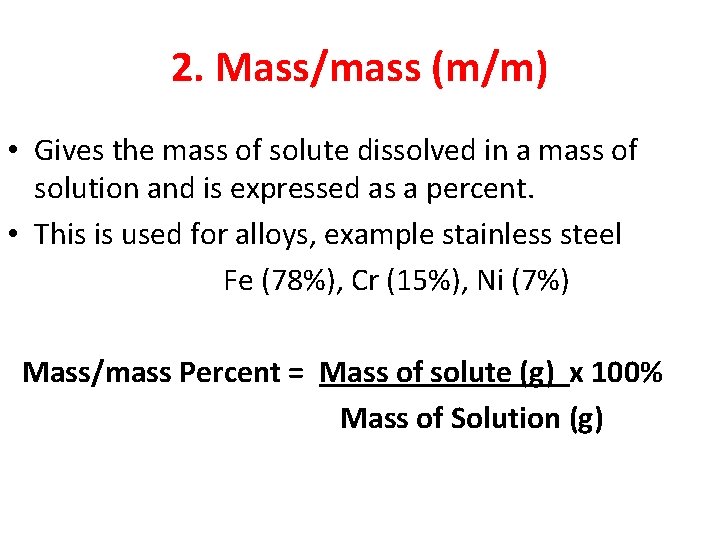
2. Mass/mass (m/m) • Gives the mass of solute dissolved in a mass of solution and is expressed as a percent. • This is used for alloys, example stainless steel Fe (78%), Cr (15%), Ni (7%) Mass/mass Percent = Mass of solute (g) x 100% Mass of Solution (g)

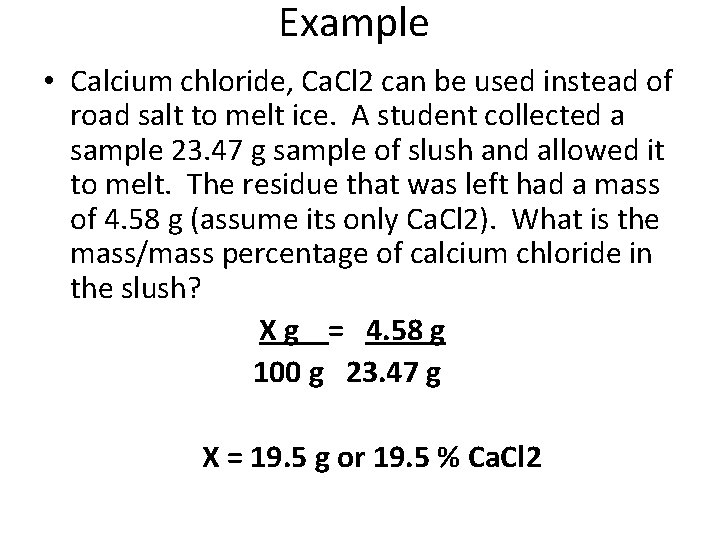
Example • Calcium chloride, Ca. Cl 2 can be used instead of road salt to melt ice. A student collected a sample 23. 47 g sample of slush and allowed it to melt. The residue that was left had a mass of 4. 58 g (assume its only Ca. Cl 2). What is the mass/mass percentage of calcium chloride in the slush? X g = 4. 58 g 100 g 23. 47 g X = 19. 5 g or 19. 5 % Ca. Cl 2

3. Volume/volume (v/v) • When mixing 2 liquids together to form a solution, it is easier to measure their volumes rather than their masses. Volume/volume %= Volume of solute (m. L) x 100% Volume of Solution (m. L)

Example • Rubbing alcohol is commonly used as an antiseptic for small cuts. It is sold as 70% v/v solution of isopropyl alcohol in water. What is the volume of isopropyl alcohol used to make 500 m. L of rubbing alcohol? v/v %= Volume of solute (m. L) x 100% Volume of Solution (m. L) 70 % = Volume of solute (m. L) x 100% 500 m. L Volume of solute (m. L) = 70% 500 m. L = 350 m. L is needed 100%

4. Parts per million (ppm) and 5. Parts per billion (ppb) • The concentration of a very small quantity of a substance in the human body or the environment is usually expressing in ppm (or ppb, next slide)

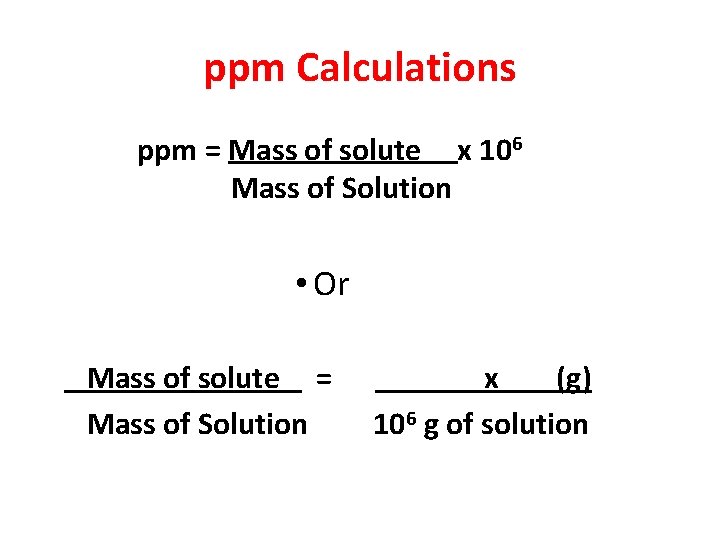
ppm Calculations ppm = Mass of solute x 106 Mass of Solution • Or Mass of solute = Mass of Solution x (g) 106 g of solution

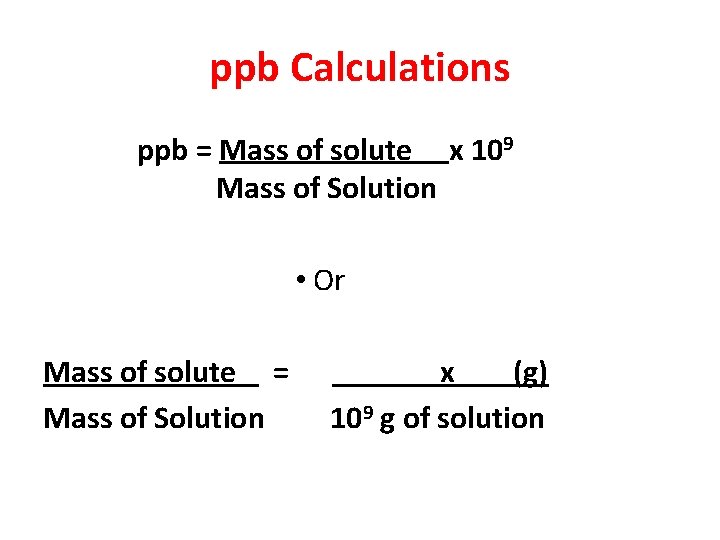
ppb Calculations ppb = Mass of solute x 109 Mass of Solution • Or Mass of solute = Mass of Solution x (g) 109 g of solution

6. Molar Concentration • The most useful and widely used in chemistry • Molar concentration is the number of moles of solute that can dissolve in 1 L of solution

• Is this a molar solution? Ouch!!

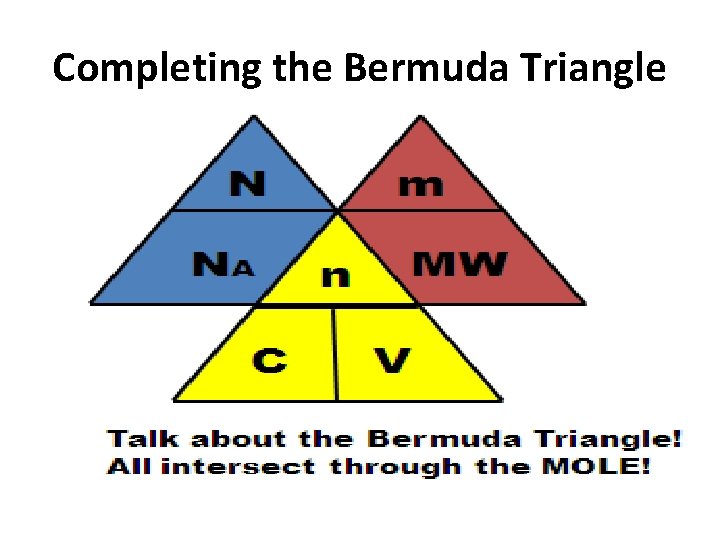
Completing the Bermuda Triangle
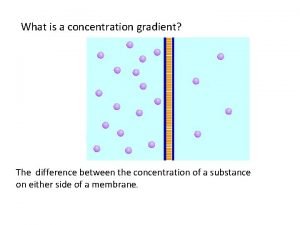
 Whats a concentration gradient
Whats a concentration gradient Movement of high concentration to low concentration
Movement of high concentration to low concentration Solutes and solvents grade 7 science
Solutes and solvents grade 7 science Adding water
Adding water Lời thề hippocrates
Lời thề hippocrates Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Chụp phim tư thế worms-breton
Chụp phim tư thế worms-breton đại từ thay thế
đại từ thay thế Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Dot
Dot Bổ thể
Bổ thể Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ Phản ứng thế ankan
Phản ứng thế ankan