7 1 Naming Ions Monatomic Ions How are















- Slides: 36

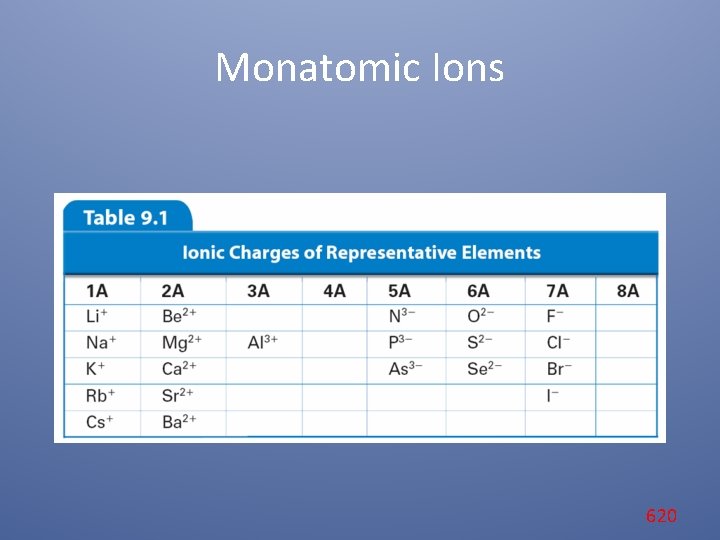
7. 1 Naming Ions Monatomic Ions: How are the charges of Group A metal and nonmetal ions related to their positions in the periodic table? Monatomic ions consist of a single atom with a positive or negative charge resulting from the loss or gain of one or more valence electrons, respectively. 608

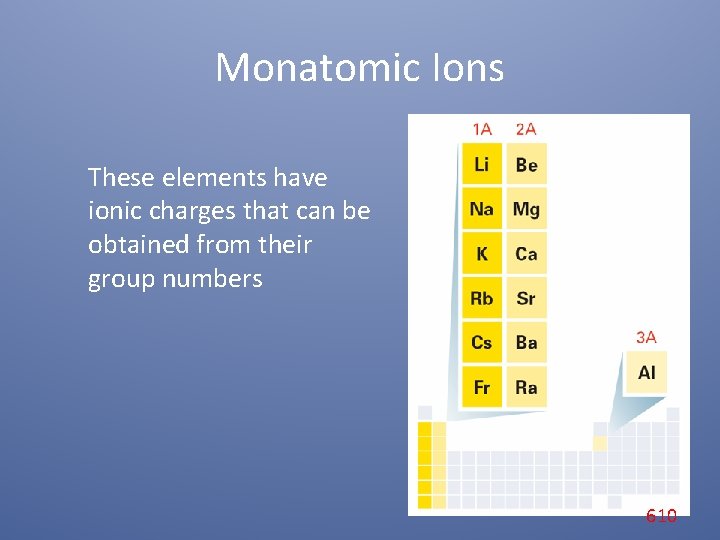
Monatomic Ions Cations When the metals in Groups 1 A, 2 A, and 3 A lose electrons, they form cations with positive charges equal to their group number. The names of the cations of the Group 1 A, Group 2 A, and Group 3 A metals are the same as the name of the metal, followed by the word ion or cation. 609

Monatomic Ions These elements have ionic charges that can be obtained from their group numbers 610

Monatomic Ions Anions The charge of any ion of a Group A nonmetal is determined by subtracting 8 from the group number. Anion names start with the stem of the element name and end in -ide. These Group A elements form anions. 611

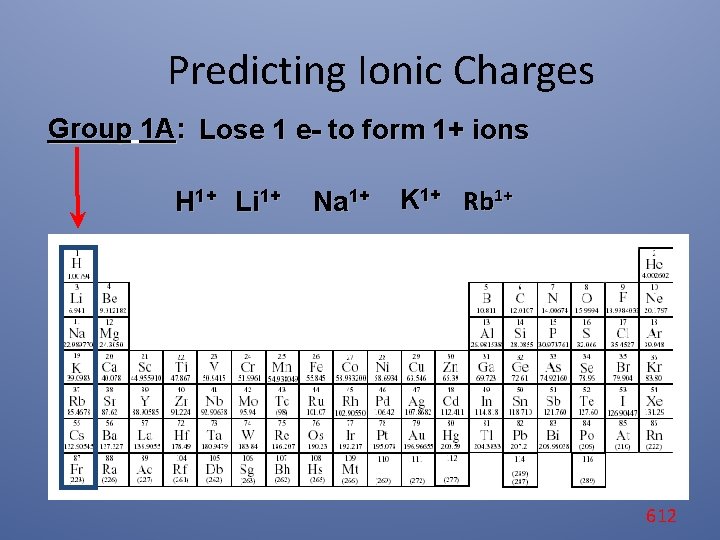
Predicting Ionic Charges Group 1 A: Lose 1 e- to form 1+ ions H 1+ Li 1+ Na 1+ K 1+ Rb 1+ 612

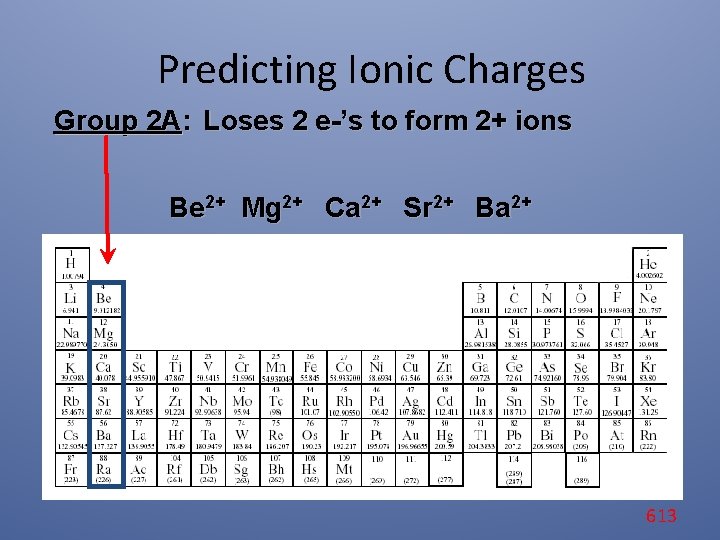
Predicting Ionic Charges Group 2 A: Loses 2 e-’s to form 2+ ions Be 2+ Mg 2+ Ca 2+ Sr 2+ Ba 2+ 613

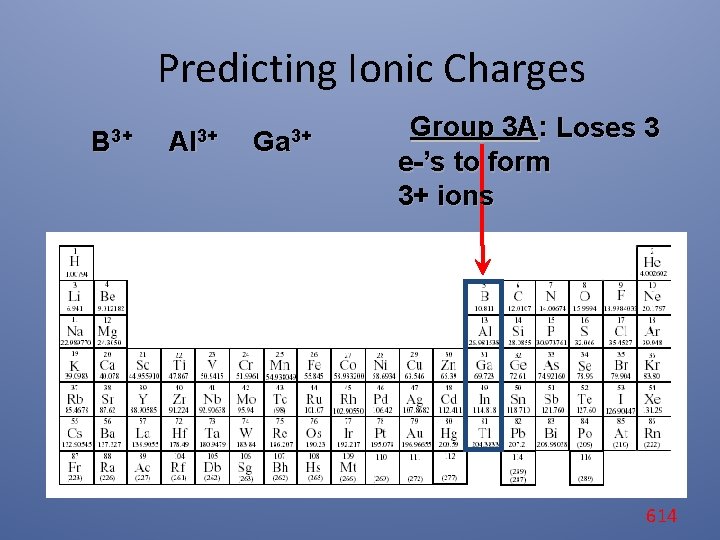
Predicting Ionic Charges B 3+ Al 3+ Ga 3+ Group 3 A: Loses 3 e-’s to form 3+ ions 614

Predicting Ionic Charges Neither! Group 4 A elements rarely form ions (they tend to share) Group 4 A: Do they lose 4 e-’s or gain 4 e-’s? 615

Predicting Ionic Charges N 3 - Nitride P 3 - Phosphide As 3 - Arsenide Group 5 A: Gains 3 e-’s to form 3 - ions 616

Predicting Ionic Charges O 2 - Oxide S 2 - Sulfide Se 2 - Selenide Group 6 A: Gains 2 e-’s to form 2 - ions 617

Predicting Ionic Charges F 1 - Fluoride Cl 1 - Chloride Group 7 A: Gains Br 1 - Bromide 1 e- to form I 1 - Iodide 1 - ions 618

Predicting Ionic Charges Group 8 A: Stable noble gases do not form ions! 619

Monatomic Ions 620

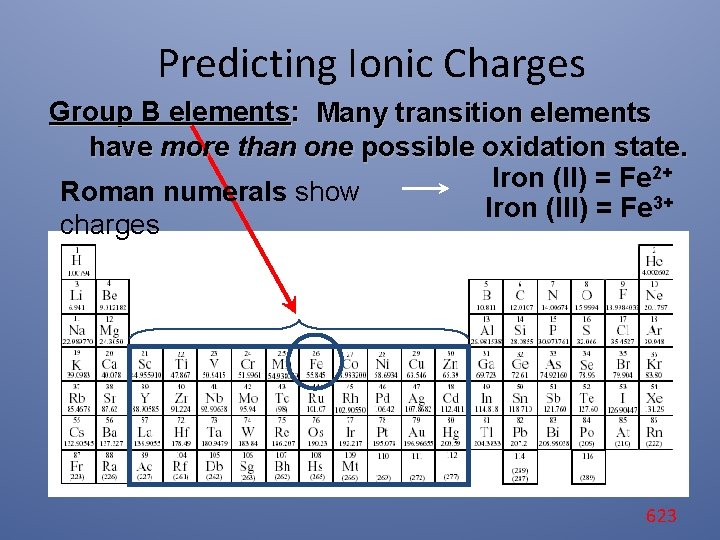
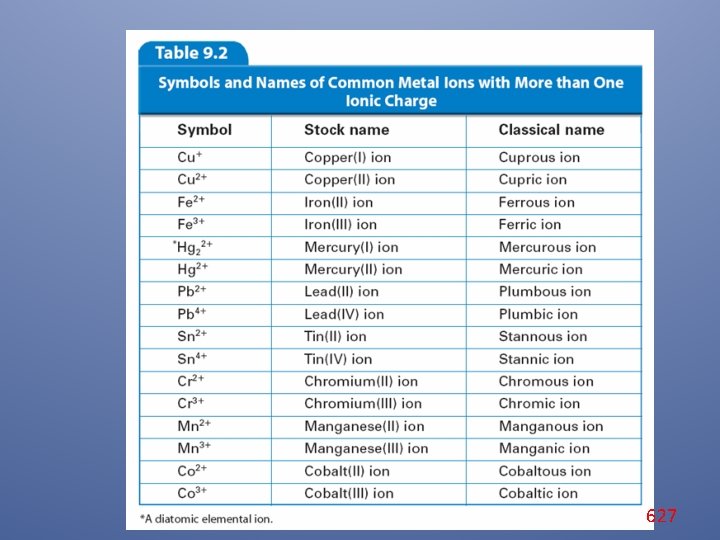
Monatomic Ions of Transition Metals How are the charges of some transition metal ions determined? The charges of the cations of many transition metal ions must be determined from the number of electrons lost. 621

Monatomic Ions There are two methods are used to name the ions of transition metals: the Stock system and the classical method In the Stock system, a Roman numeral in parentheses is placed after the name of the element to indicate the numerical value of the charge. (Cu+ is copper (I) ion) In an older less, useful method, the classical name of the element is used to form the root name for the element. (Cu+ is cuprous ion) 622

Predicting Ionic Charges Group B elements: Many transition elements have more than one possible oxidation state. 2+ Iron (II) = Fe Roman numerals show Iron (III) = Fe 3+ charges 623

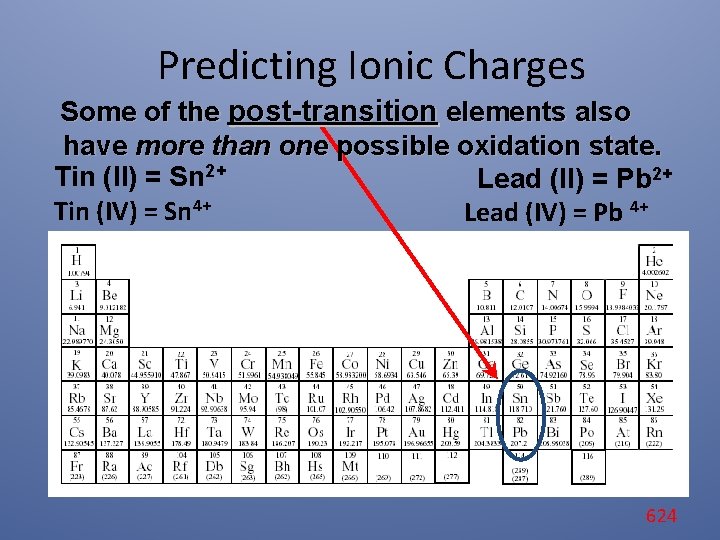
Predicting Ionic Charges Some of the post-transition elements also have more than one possible oxidation state. Tin (II) = Sn 2+ Lead (II) = Pb 2+ Tin (IV) = Sn 4+ Lead (IV) = Pb 4+ 624

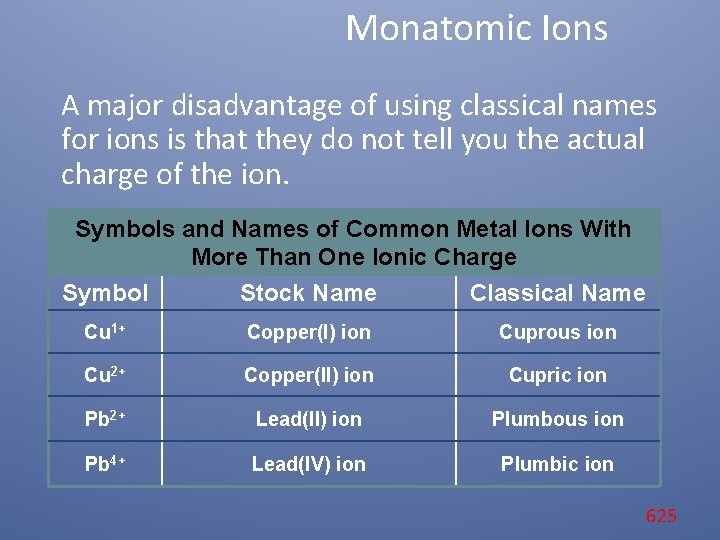
Monatomic Ions A major disadvantage of using classical names for ions is that they do not tell you the actual charge of the ion. Symbols and Names of Common Metal Ions With More Than One Ionic Charge Symbol Stock Name Classical Name Cu 1+ Copper(I) ion Cuprous ion Cu 2+ Copper(II) ion Cupric ion Pb 2+ Lead(II) ion Plumbous ion Pb 4+ Lead(IV) ion Plumbic ion 625

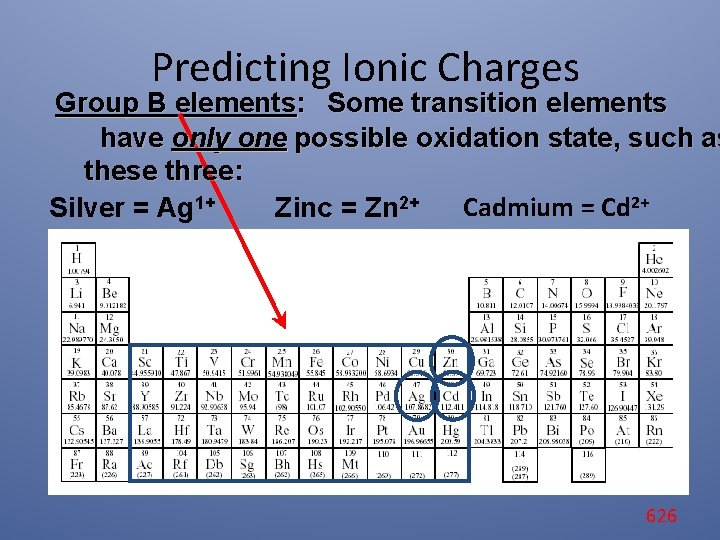
Predicting Ionic Charges Group B elements: Some transition elements have only one possible oxidation state, such as these three: Cadmium = Cd 2+ Silver = Ag 1+ Zinc = Zn 2+ 626

627

Conceptual Problem 9. 1 a) K+, cation, potassium ion b) I-, anion, iodide ion c) S 2 -, anion, sulfide ion d) Pb 4+, cation, lead (IV) ion 628

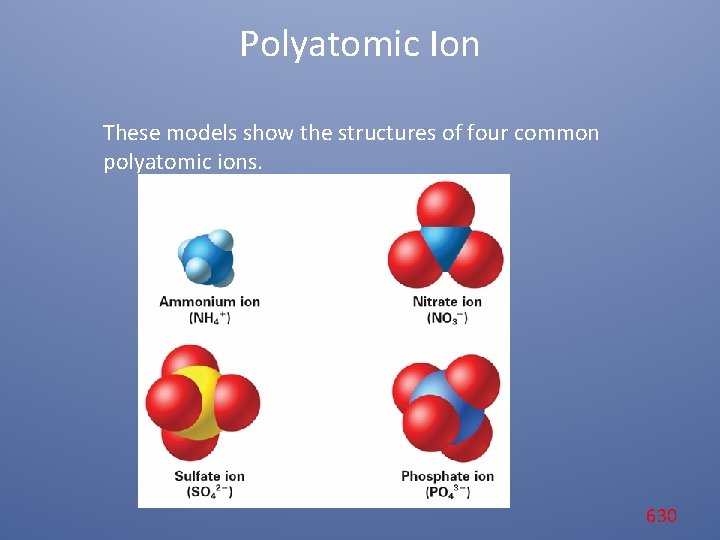
Polyatomic Ions What are the two endings of the names of most polyatomic ions? Some ions, called polyatomic ions, are composed of more than one atom. The names of most polyatomic anions end in ite or -ate. 629

Polyatomic Ion These models show the structures of four common polyatomic ions. 630

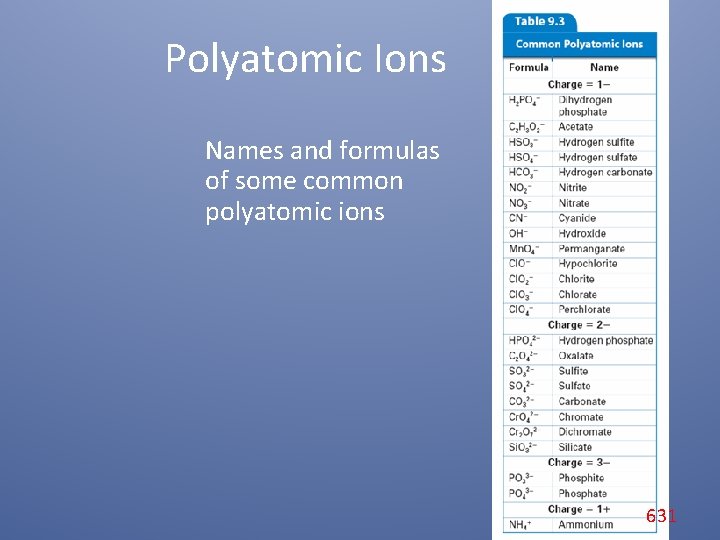
Polyatomic Ions Names and formulas of some common polyatomic ions 631

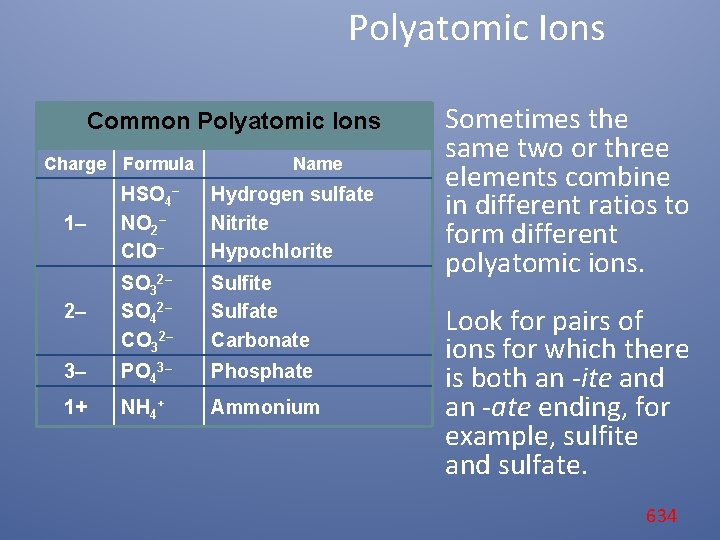
Polyatomic Ions Common Polyatomic Ions Charge Formula Name 1– HSO 4– NO 2– Cl. O– Hydrogen sulfate Nitrite Hypochlorite 2– SO 32– SO 42– CO 32– Sulfite Sulfate Carbonate 3– PO 43– Phosphate 1+ NH 4+ Ammonium The names and formulas of some common polyatomic ions are shown here. Note that the names of most polyatomic ions end in -ite or -ate. 632

Polyatomic Ions Common Polyatomic Ions Charge Formula Name 1– HSO 4– NO 2– Cl. O– Hydrogen sulfate Nitrite Hypochlorite 2– SO 32– SO 42– CO 32– Sulfite Sulfate Carbonate 3– PO 43– Phosphate 1+ NH 4+ Ammonium For example, notice the endings of the names of the hypochlorite ion (Cl. O–) and the carbonate ion (CO 3–). 633

Polyatomic Ions Common Polyatomic Ions Charge Formula Name 1– HSO 4– NO 2– Cl. O– Hydrogen sulfate Nitrite Hypochlorite 2– SO 32– SO 42– CO 32– Sulfite Sulfate Carbonate 3– PO 43– Phosphate 1+ NH 4+ Ammonium Sometimes the same two or three elements combine in different ratios to form different polyatomic ions. Look for pairs of ions for which there is both an -ite and an -ate ending, for example, sulfite and sulfate. 634

Polyatomic Ions Note the number of oxygen atoms and the endings on each name. You should be able to discern a pattern in the naming convention. -ite -ate SO 32−, sulfite SO 42–, sulfate NO 2–, nitrite NO 3–, nitrate Cl. O 2–, chlorite Cl. O 3–, chlorate 635

Polyatomic Ions – The charge is the same on each polyatomic ion in a pair for which there is both an -ite and an -ate ion. – The -ite ending indicates one less oxygen atom than the -ate ending. – However, the ending does not tell you the actual number of oxygen atoms in the ion. • For example, the nitrite ion has two oxygen atoms, and the sulfite ion has three oxygen atoms. 636

Practice by naming these: • • • Na 1+ Ca 2+ Al 3+ Fe 3+ Pb 2+ Li 1+ 637

Write symbols for these: • Potassium ion • Magnesium ion • Copper (II) ion • Chromium (VI) ion • Barium ion • Mercury (II) ion 638

Naming Anions • Change monatomic element ending to – ide 1 • F a Fluorine atom becomes Fluoride ion. 639

Practice by naming these: • Cl 1 • N 31 • Br 2 • O 640

Write symbols for these: • Sulfide ion • Iodide ion • Phosphide ion 641

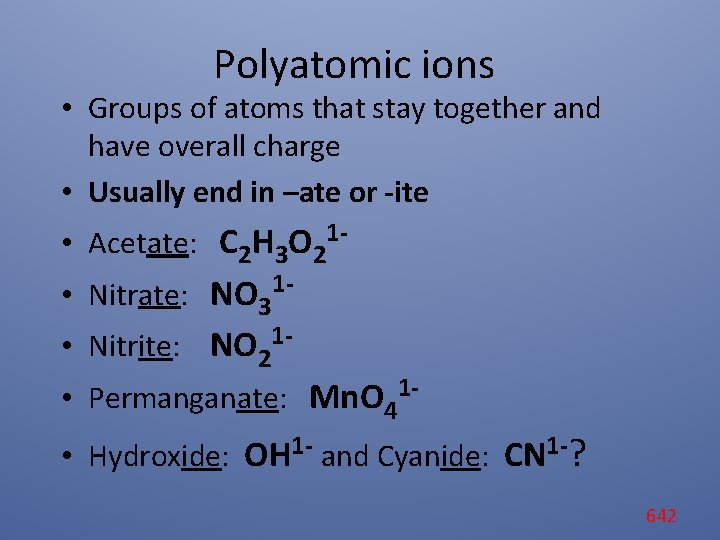
Polyatomic ions • Groups of atoms that stay together and have overall charge • Usually end in –ate or -ite • Acetate: C 2 H 3 O 21 • Nitrate: NO 31 - • Nitrite: NO 21 • Permanganate: Mn. O 41 • Hydroxide: OH 1 - and Cyanide: CN 1 -? 642

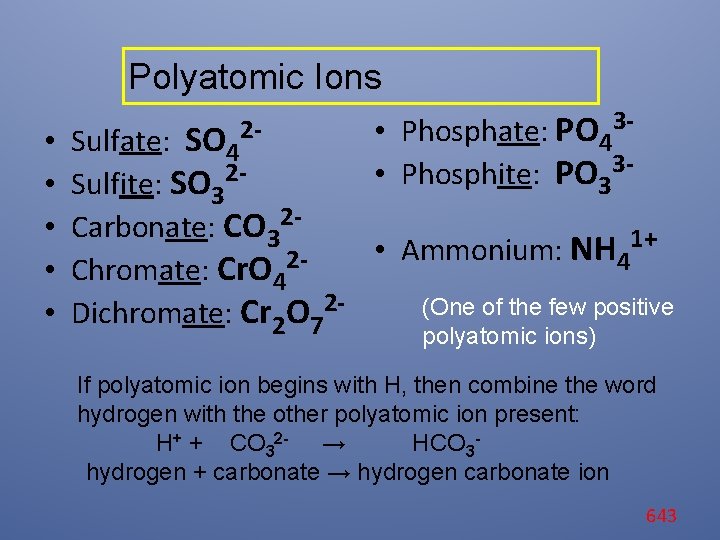
Polyatomic Ions • • • 2 - • Phosphate: PO 433 • Phosphite: PO 3 Sulfate: SO 4 Sulfite: SO 32 Carbonate: CO 321+ • Ammonium: NH 24 Chromate: Cr. O 4 (One of the few positive Dichromate: Cr 2 O 72 polyatomic ions) If polyatomic ion begins with H, then combine the word hydrogen with the other polyatomic ion present: H+ + CO 32 - → HCO 3 hydrogen + carbonate → hydrogen carbonate ion 643