1 2 Monatomic Elements Very Weak Attractions LO
- Slides: 8

1. 2 Monatomic Elements Very Weak Attractions LO: I know about the weak forces of attraction between atoms of the noble gases.

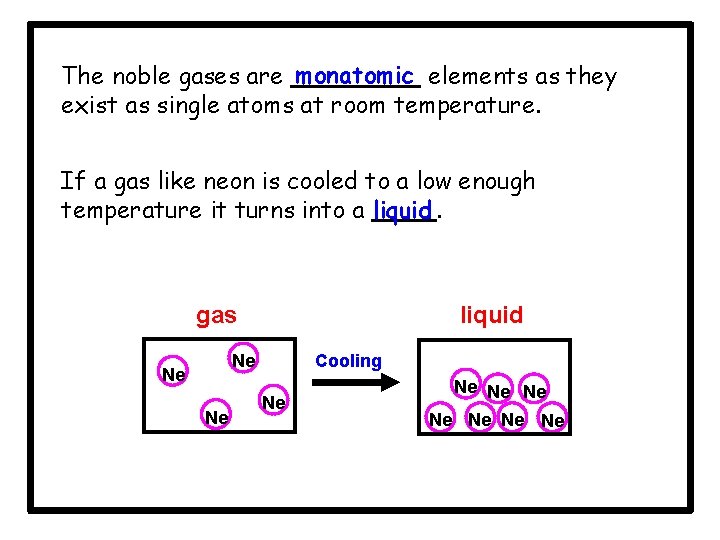
The noble gases are monatomic elements as they exist as single atoms at room temperature. If a gas like neon is cooled to a low enough temperature it turns into a liquid. gas liquid Cooling Ne Ne Ne

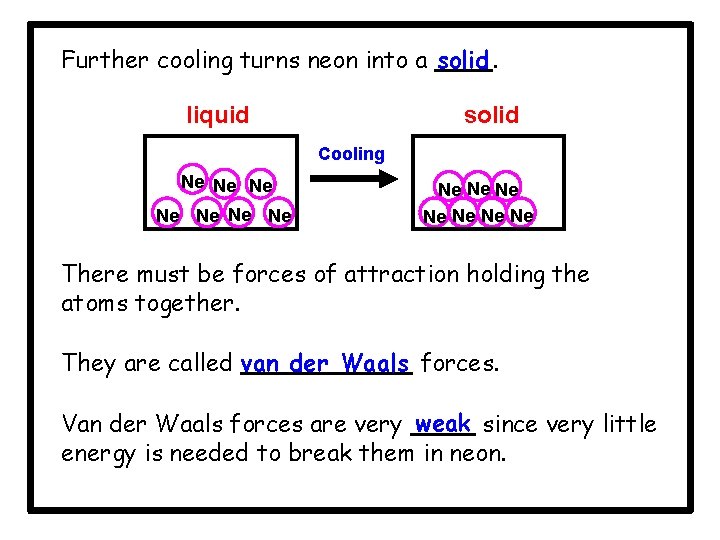
Further cooling turns neon into a solid. liquid solid Cooling Ne Ne Ne Ne There must be forces of attraction holding the atoms together. They are called van der Waals forces. Van der Waals forces are very weak since very little energy is needed to break them in neon.

Van der Waals Forces LO: I understand how van der Waals forces come about.

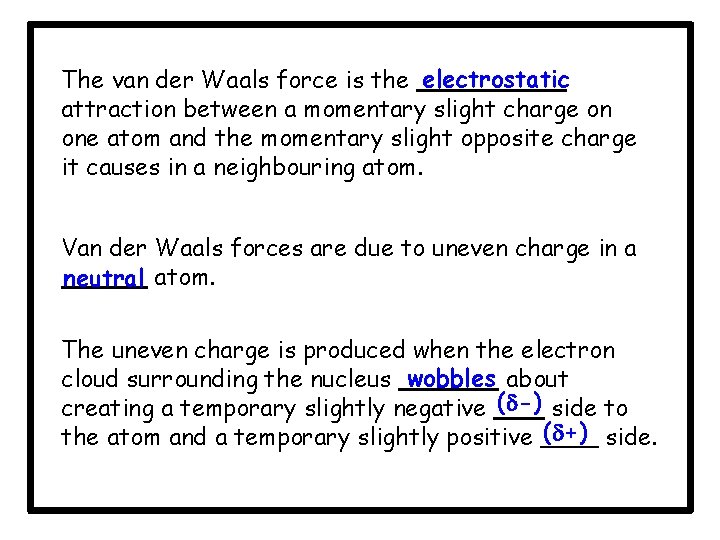
The van der Waals force is the electrostatic attraction between a momentary slight charge on one atom and the momentary slight opposite charge it causes in a neighbouring atom. Van der Waals forces are due to uneven charge in a neutral atom. The uneven charge is produced when the electron cloud surrounding the nucleus wobbles about creating a temporary slightly negative ( -) side to the atom and a temporary slightly positive ( +) side.

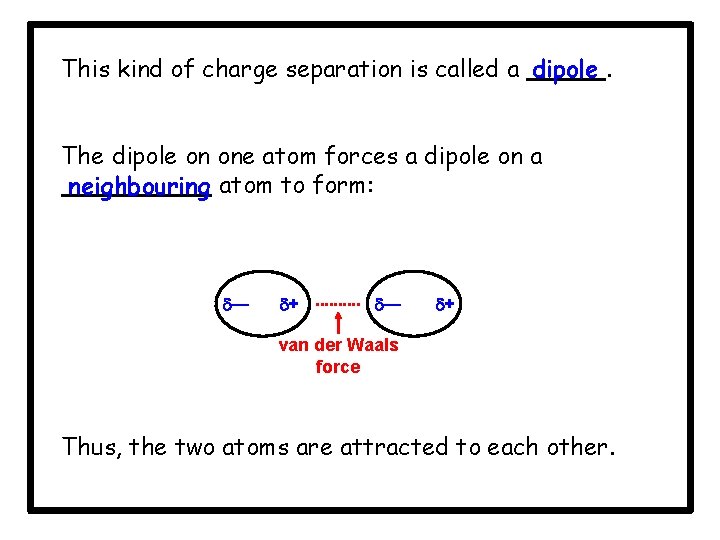
This kind of charge separation is called a dipole. The dipole on one atom forces a dipole on a neighbouring atom to form: — + van der Waals force Thus, the two atoms are attracted to each other.

The Noble Gas Series LO: I understand how van der Waals forces affect the boiling points of the noble gases.

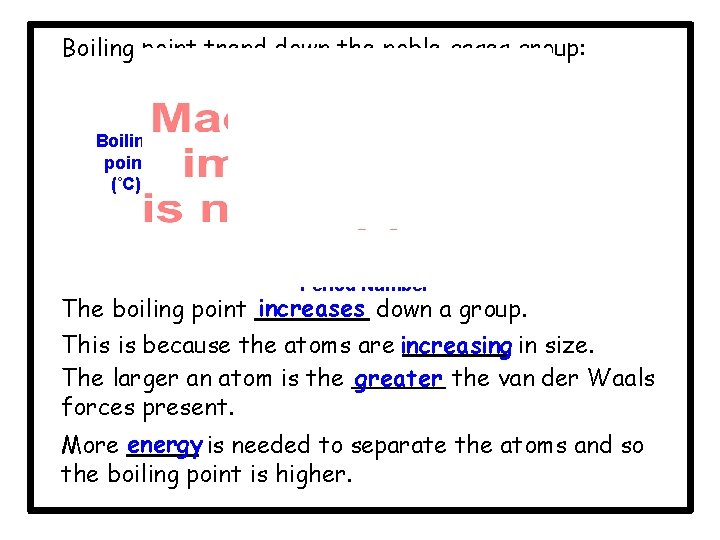
Boiling point trend down the noble gases group: Ra Xe Boiling point (˚C) Ar He Kr Ne Period Number The boiling point increases down a group. This is because the atoms are increasing in size. The larger an atom is the greater the van der Waals forces present. More energy is needed to separate the atoms and so the boiling point is higher.