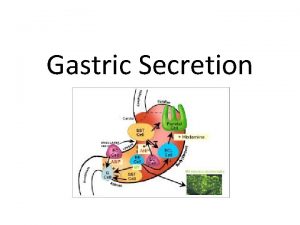
19982009 Korean Guideline Indications Definite indications 19982009 Peptic




























- Slides: 50


1998/2009 Korean Guideline Indications • Definite indications (1998/2009) – Peptic ulcer (including scar) – Marginal zone B-cell lymphoma – EGC, after endoscopic resection • Recommended indications (2009) – 1° relatives of gastric cancer – Unexplained IDA – Chronic ITP • Possible indications (2009) – Atrophic gastritis – Non-ulcer dyspepsia – Long-term use of NSAID 김나영 등. 헬리코박터 파일로리 감염의 진단 및 치료 가이드라인. 대한소화기학회지 2009; 54: 269 -278.

2013 Korean guideline indications • • Peptic ulcer (1 A) Marginal zone B cell lymphoma (1 A) EGC, after endoscopic resection (1 A) ITP (1 A) Long-term aspirin use with peptic ulcer history (2 C) Atrophic gastritis/intestinal metaplasia (2 C) Family history of gastric cancer (2 B) Functional dyspepsia (2 A) Kim SG et al. J Gastroenterol Hepatol 2014; 29: 1371 -1386.

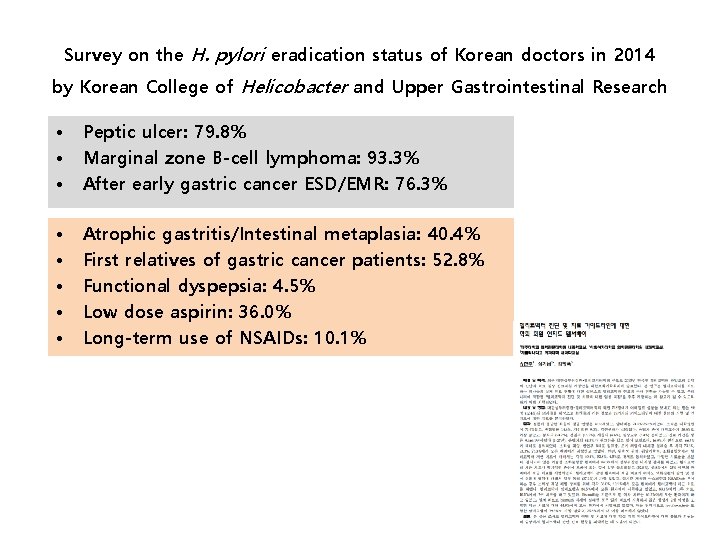
Survey on the H. pylori eradication status of Korean doctors in 2014 by Korean College of Helicobacter and Upper Gastrointestinal Research • • • Peptic ulcer: 79. 8% Marginal zone B-cell lymphoma: 93. 3% After early gastric cancer ESD/EMR: 76. 3% • • • Atrophic gastritis/Intestinal metaplasia: 40. 4% First relatives of gastric cancer patients: 52. 8% Functional dyspepsia: 4. 5% Low dose aspirin: 36. 0% Long-term use of NSAIDs: 10. 1%




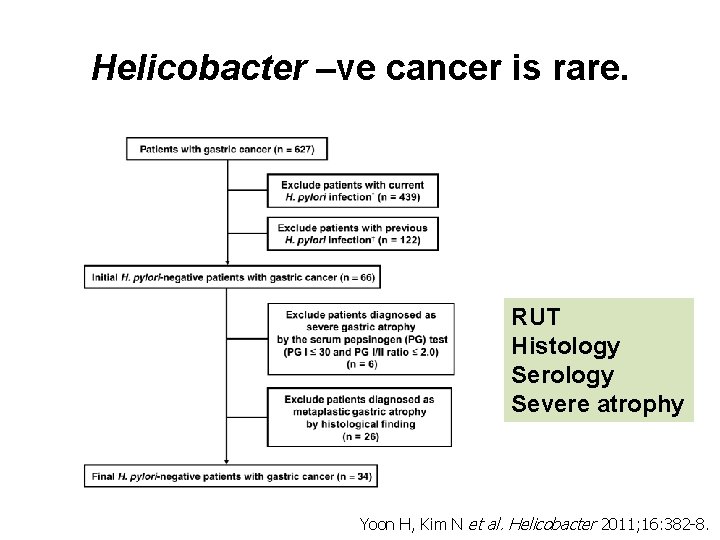
Helicobacter –ve cancer is rare. RUT Histology Serology Severe atrophy Yoon H, Kim N et al. Helicobacter 2011; 16: 382 -8.

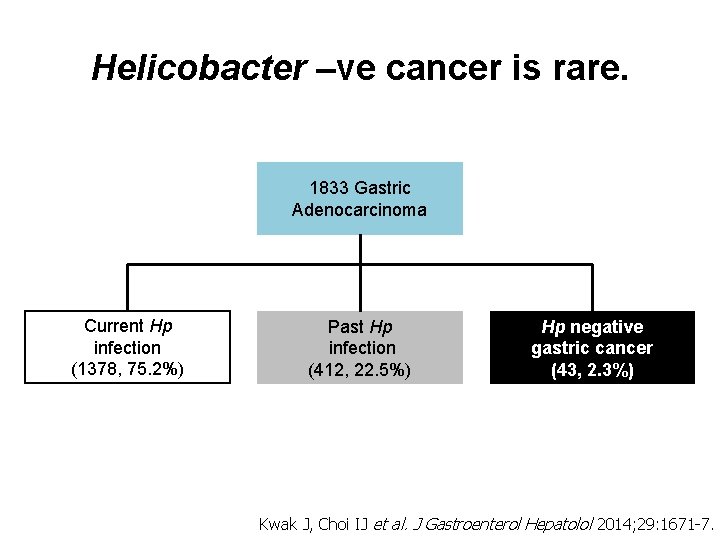
Helicobacter –ve cancer is rare. 1833 Gastric Adenocarcinoma Current Hp infection (1378, 75. 2%) Past Hp infection (412, 22. 5%) Hp negative gastric cancer (43, 2. 3%) Kwak J, Choi IJ et al. J Gastroenterol Hepatolol 2014; 29: 1671 -7.

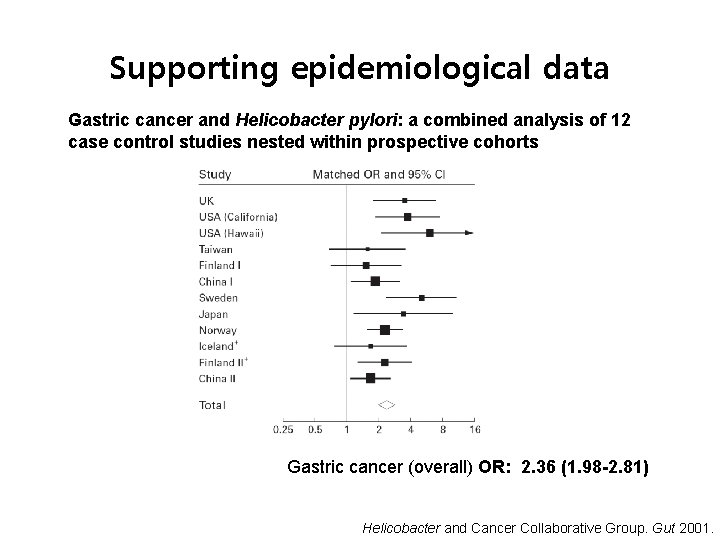
Supporting epidemiological data Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts Gastric cancer (overall) OR: 2. 36 (1. 98 -2. 81) Helicobacter and Cancer Collaborative Group. Gut 2001.

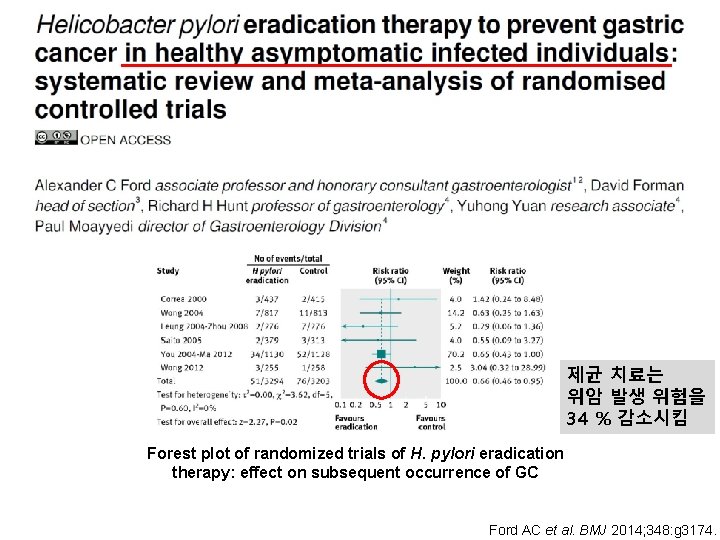
제균 치료는 위암 발생 위험을 34 % 감소시킴 Forest plot of randomized trials of H. pylori eradication therapy: effect on subsequent occurrence of GC Ford AC et al. BMJ 2014; 348: g 3174.

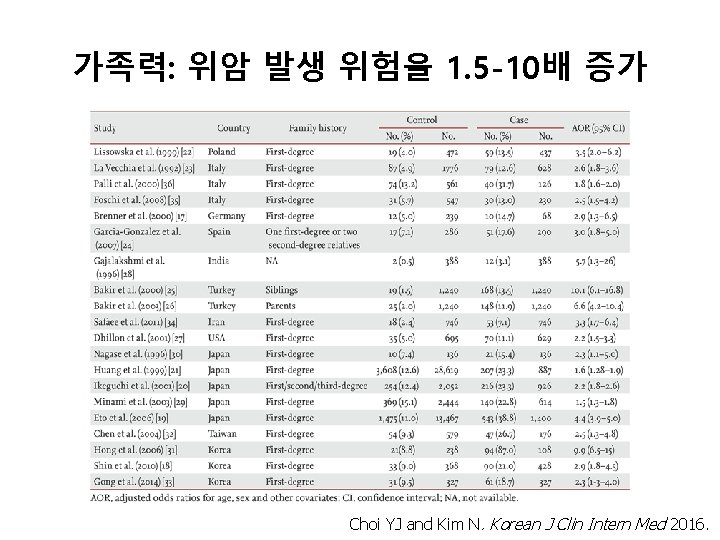
가족력: 위암 발생 위험을 1. 5 -10배 증가 Choi YJ and Kim N. Korean J Clin Intern Med 2016.

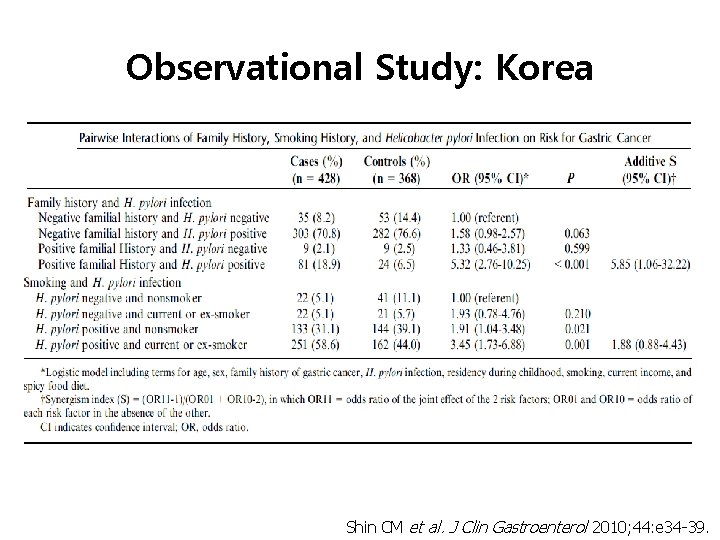
Observational Study: Korea Shin CM, Kim N et al. J Clin Gastroenterol 2010; 44: e 34 -39.

Observational Study: Korea Shin CM et al. J Clin Gastroenterol 2010; 44: e 34 -39.

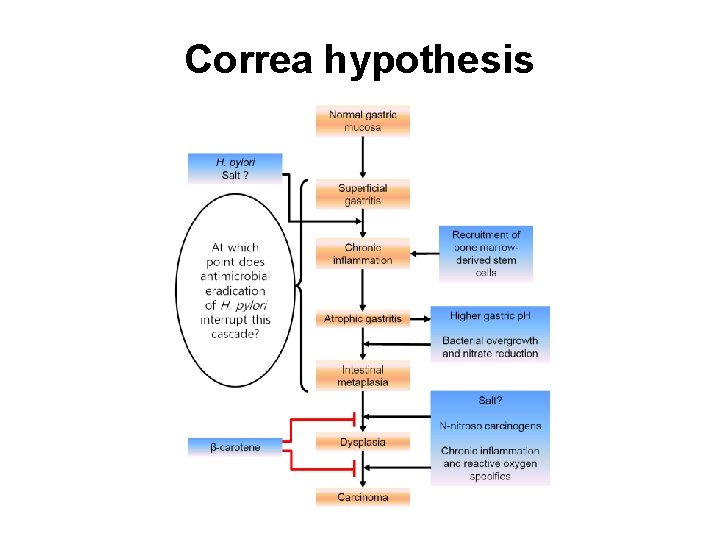
Correa hypothesis

Point of no return theory Effect of early eradication on Helicobacter pylori related gastric carcinogenesis in Mongolian gerbils Nozaki K et al. Cancer Sci 2003; 94: 235 -239. Helicobacter felis eradication restores normal architecture and inhibits gastric cancer progression in C 57 BL/6 Mice Cai X et al. Gastroenterology 2005; 128: 1937 -1952. Helicobacter pylori eradication to prevent gastric cancer in a highrisk region of China: a randomized controlled trial. Wong BC et al. JAMA 2004; 291: 187– 194.

Overall population Subjects without precancerous lesions Wong et al. JAMA 2004.

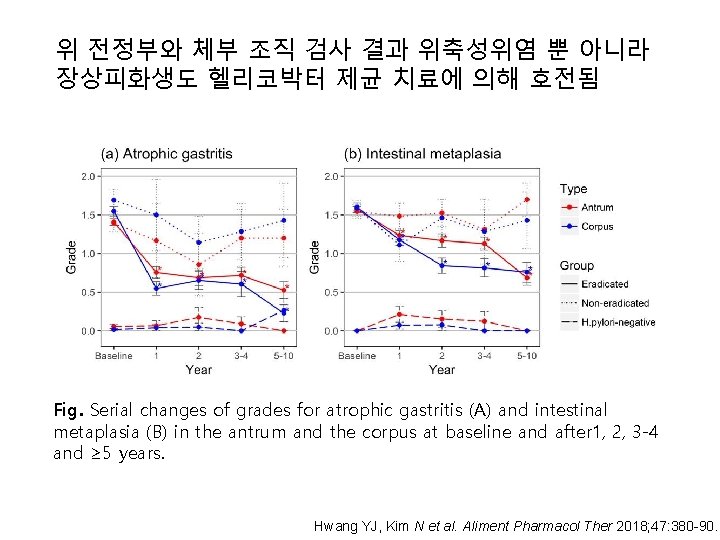
위 전정부와 체부 조직 검사 결과 위축성위염 뿐 아니라 장상피화생도 헬리코박터 제균 치료에 의해 호전됨 Fig. Serial changes of grades for atrophic gastritis (A) and intestinal metaplasia (B) in the antrum and the corpus at baseline and after 1, 2, 3 -4 and ≥ 5 years. Hwang YJ, Kim N et al. Aliment Pharmacol Ther 2018; 47: 380 -90.

Hwang YJ, Kim N et al. Aliment Pharmacol Ther 2018; 47: 380 -90.


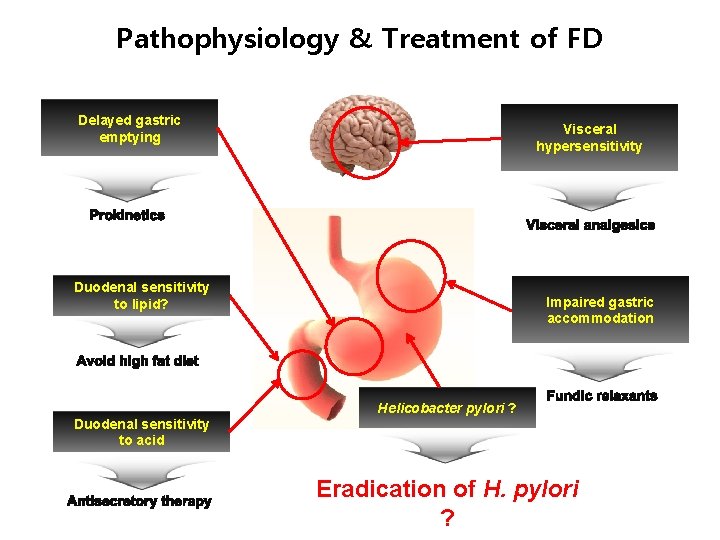
Pathophysiology & Treatment of FD Delayed gastric emptying Visceral hypersensitivity Duodenal sensitivity to lipid? Impaired gastric accommodation Helicobacter pylori ? Duodenal sensitivity to acid Eradication of H. pylori ?

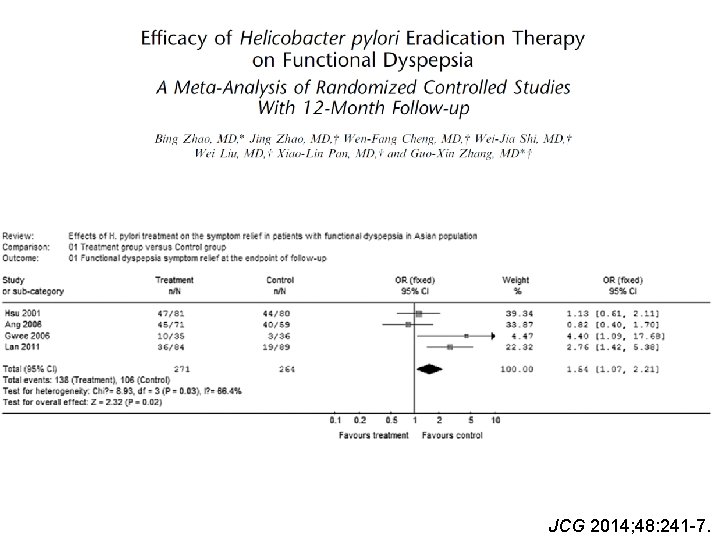
JCG 2014; 48: 241 -7.

JCG 2014; 48: 241 -7.

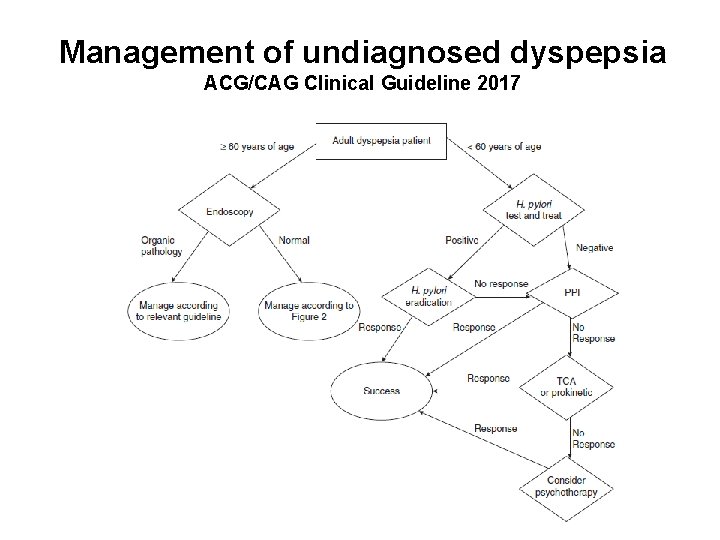
Management of undiagnosed dyspepsia ACG/CAG Clinical Guideline 2017

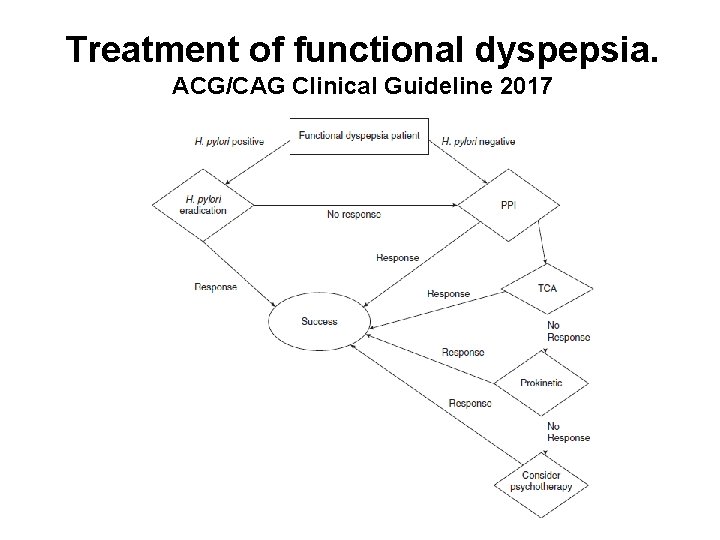
Treatment of functional dyspepsia. ACG/CAG Clinical Guideline 2017

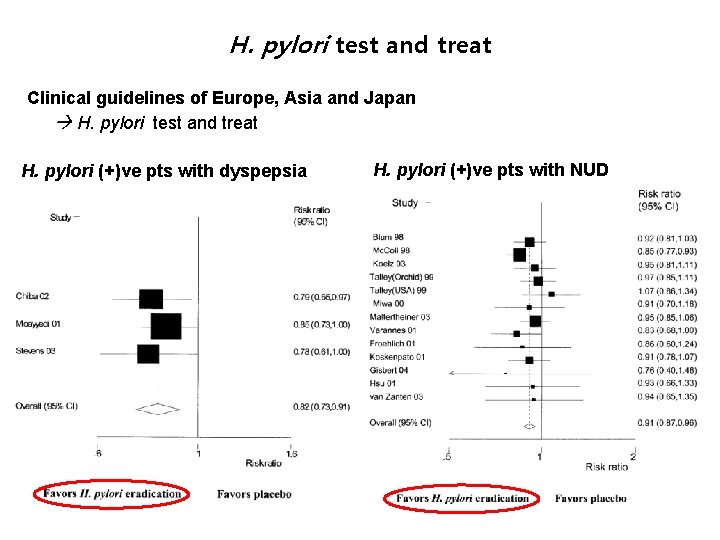
H. pylori test and treat Clinical guidelines of Europe, Asia and Japan H. pylori test and treat H. pylori (+)ve pts with dyspepsia H. pylori (+)ve pts with NUD


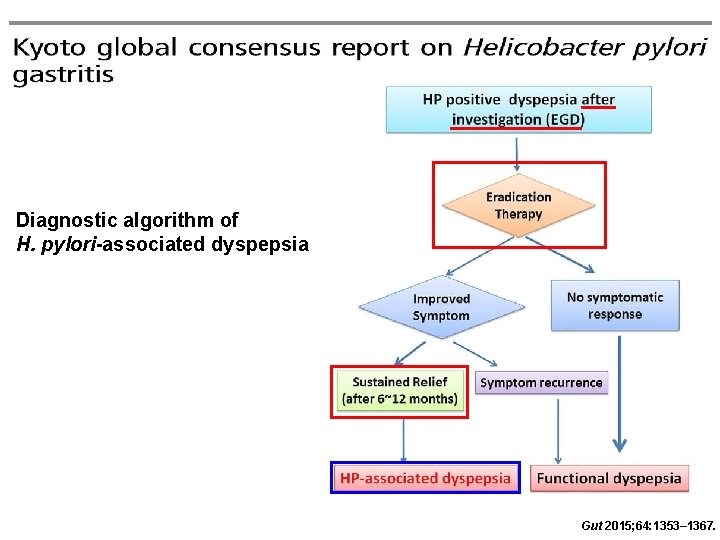
Diagnostic algorithm of H. pylori-associated dyspepsia Gut 2015; 64: 1353– 1367.

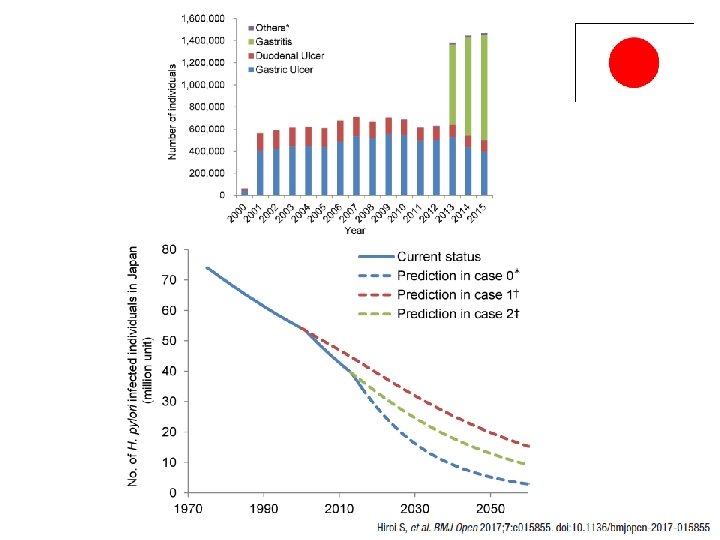
일본의 헬리코박터 제균 정책 February 21, 2013 Helicobacter pylori gastritis (헬리코박터 위염) has been approved by Japan’s Ministry of Health, Labour and Welfare as an additional indication for H. pylori eradication by triple therapy with proton pump inhibitors. 제균치료를 통해 감염자에게는 위암 위험성을, 주변 비 감염자들에게는 전염 기회를 줄여 20 -30년 후에는 서구 선진국의 위암 유병률과 헬리코박터 감염률 수준으로 낮추겠다는 것이 목표임 Lee SY. Korean J Gastroenterol 2014; 63: 151 -7.


제균치료 약제 2009년 가이드라인 1 st line (표준 3제 요법) PPI (standard dose bid) + clarithromycin (0. 5 g bid) + amoxicillin (1 g bid) for 1 or 2 weeks 2 nd line (4제 요법) PPI (standard dose bid) + metronidazole (0. 5 g tid)+bismuth (120 mg qid)+tetracycline (0. 5 g qid) for 1 or 2 weeks Therapy for a week or two weeks issue is not yet clear, so it is need to clarify the treatment duration through evidence of current or future. 김나영 등. 헬리코박터 파일로리 감염의 진단 및 치료 가이드라인. 대한소화기학회지 2009; 54: 269 -278.

제균치료 약제 2013년 가이드라인 1 st line PPI (standard dose bid) + clarithromycin (0. 5 g bid) + amoxicillin (1 g bid) for 1 weeks High Clarithromycin Resistance PPI (standard dose bid)+metronidazole (0. 5 g tid)+bismuth (120 mg qid)+tetracycline (0. 5 g qid) for 1 or 2 weeks 2 nd line If the triple therapy fail as 1 st line PPI (standard dose bid)+metronidazole (0. 5 g tid)+bismuth (120 mg qid)+tetracycline (0. 5 g qid) for 1 or 2 weeks If the quadruple therapy fail as 1 st line Combination of two/more antibiotics without prior exposures experiences Kim SG et al. J Gastroenterol Hepatol 2014; 29: 1371 -1386.

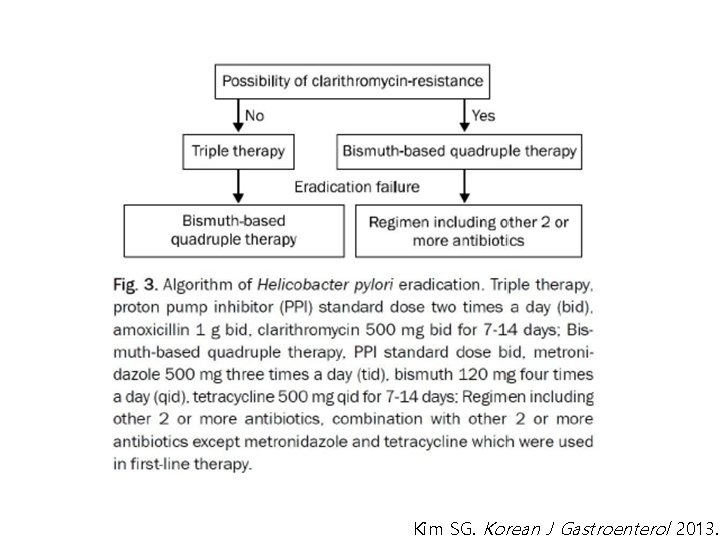
Kim SG. Korean J Gastroenterol 2013.



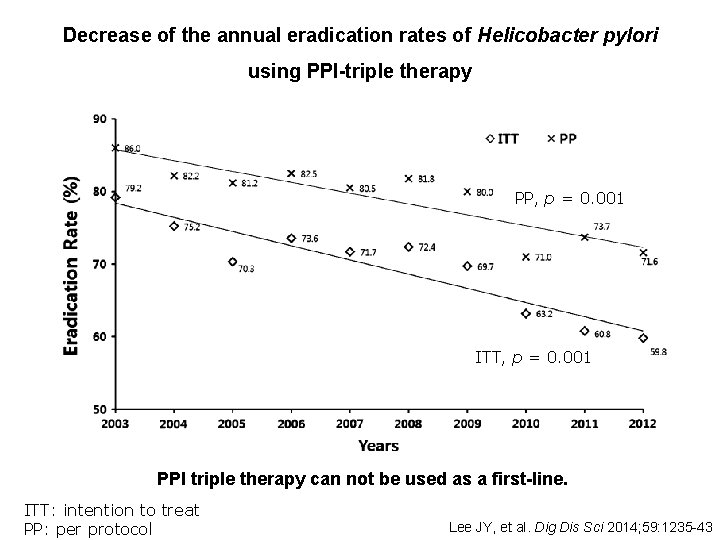
Decrease of the annual eradication rates of Helicobacter pylori using PPI-triple therapy PP, p = 0. 001 ITT, p = 0. 001 PPI triple therapy can not be used as a first-line. ITT: intention to treat PP: per protocol Lee JY, et al. Dig Dis Sci 2014; 59: 1235 -43

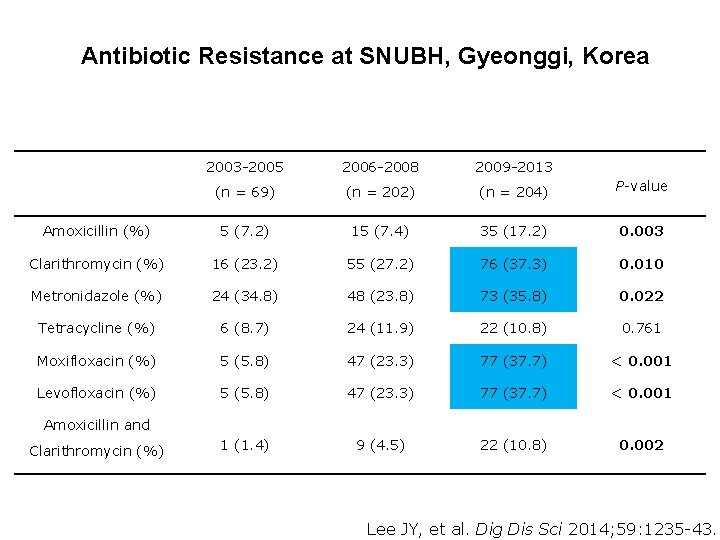
Antibiotic Resistance at SNUBH, Gyeonggi, Korea 2003 -2005 2006 -2008 2009 -2013 (n = 69) (n = 202) (n = 204) P-value Amoxicillin (%) 5 (7. 2) 15 (7. 4) 35 (17. 2) 0. 003 Clarithromycin (%) 16 (23. 2) 55 (27. 2) 76 (37. 3) 0. 010 Metronidazole (%) 24 (34. 8) 48 (23. 8) 73 (35. 8) 0. 022 Tetracycline (%) 6 (8. 7) 24 (11. 9) 22 (10. 8) 0. 761 Moxifloxacin (%) 5 (5. 8) 47 (23. 3) 77 (37. 7) < 0. 001 Levofloxacin (%) 5 (5. 8) 47 (23. 3) 77 (37. 7) < 0. 001 1 (1. 4) 9 (4. 5) 22 (10. 8) 0. 002 Amoxicillin and Clarithromycin (%) Lee JY, et al. Dig Dis Sci 2014; 59: 1235 -43.

Increase of Secondary Antibiotic Resistance Amoxicillin Clarithromycin Lee JW et al. Helicobacter 2013; 59: 1235 -43.

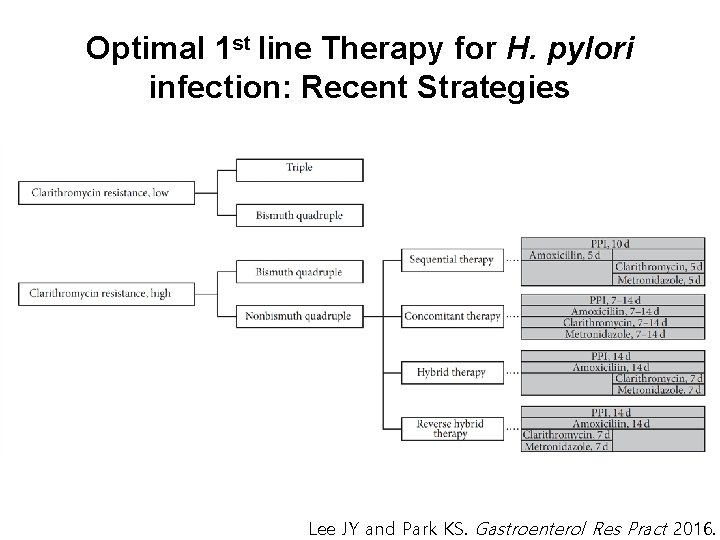
Optimal 1 st line Therapy for H. pylori infection: Recent Strategies Lee JY and Park KS. Gastroenterol Res Pract 2016.

Regimen: “five plus five” day Tx PPI ac bid Amoxicillin 1 g pc bid 5 days PPI ac bid Clarithromycin 500 mg pc bid Tinidazole (or metronidazole) 500 mg pc bid 5 days

Rationale of the Sequential Regimen • Efficacy of a triple therapy (PPI, clarithromycin, and tinidazole) was inversely related to the bacterial load (inoculum effect). • Higher eradication rates could be achieved in those with a low bacterial density in the stomach. Maconi G et al. Am J Gastroenterol 2001; 96: 359 -66. Lai YC et al. World J Gastroenterol 2004; 10: 991 -4.

Rationale of the Sequential Regimen • Initial use of amoxicillin prevent the selection of secondary clarithromycin resistance. Murakami K et al. Int J Antimicrob Agents 2002; 19: 67 -70. • Disruption of bacterial cell wall by amoxicillin prevents the development of efflux channels, which improves the efficacy of clarithromycin in the second phase of treatment. Webber MA et al. J Antimicrob Chemother 2003; 51: 9 -11. De Francesco V et al. Ann Intern Med 2006; 144: 94 -100.

Rationale of the Sequential Regimen • Improved effect with sequential regimen compared with standard triple therapy may be due to additional antibiotic use (tinidazole or metronidazole), not due to the sequential administration per se? • Sequential administration of antibiotics may promote drug resistance? Graham DY et al. Drugs 2008; 68: 725 -36.

Concomitant Therapy • Sequential therapy의 제균율 향상이 순차적인 약물 투여 때문인지 아니면 metronidazole 과 같은 항생제의 추가 적 사용 때문인지 불명확함 • 복잡하게 처방하여 순응도 저하를 보일 우려가 있기 때 문에 한꺼번에 처방 1 -5일: amoxicillin 1 g + PPI (bid) Sequential therapy Concomitant therpay 6 -10일: clarithromycin 500 mg + metronidazole 500 mg + PPI (bid) amoxicillin 1 g + clarithromycin 500 mg + metronidazole 500 mg (bid) 7 -14일

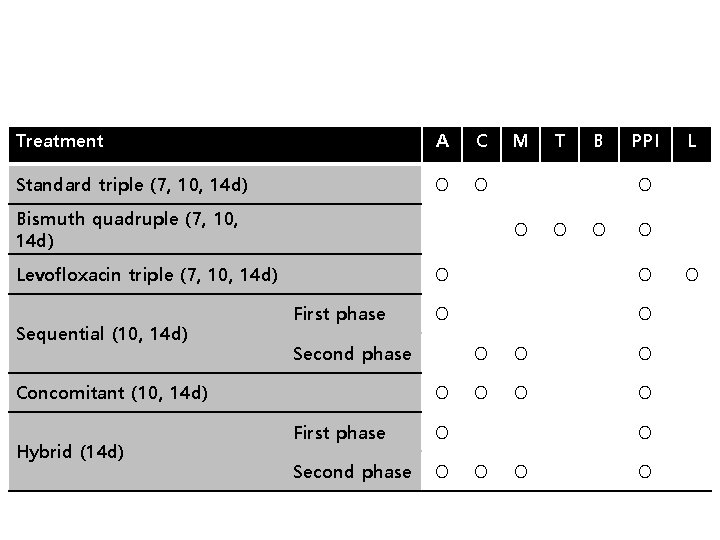
Treatment A C Standard triple (7, 10, 14 d) O O Bismuth quadruple (7, 10, 14 d) First phase Hybrid (14 d) B PPI O O O First phase O Second phase O O O O O L O O Second phase Concomitant (10, 14 d) T O O Levofloxacin triple (7, 10, 14 d) Sequential (10, 14 d) M O O O

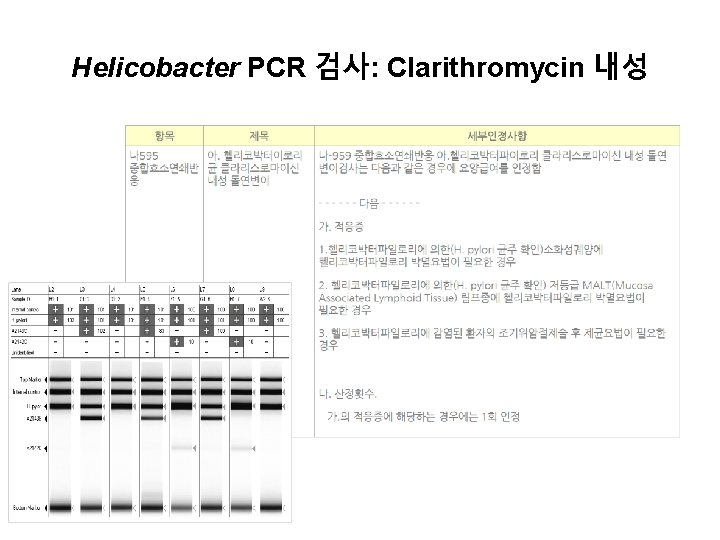
Helicobacter PCR 검사: Clarithromycin 내성

PCR positive 인 경우 1) Clarithromycin 을 metronidazole 로 변경한 삼제요법 2) Quadruple therapy 3) Sequential/Concomitant therapy



Take Home Message
 Peptic ulcer disease
Peptic ulcer disease Gastric ulcer anatomy
Gastric ulcer anatomy Arsas symptoms
Arsas symptoms Peptic ulcer diseas
Peptic ulcer diseas Peptic ulcers causes
Peptic ulcers causes What is emetics
What is emetics Objectives of peptic ulcer
Objectives of peptic ulcer Acholorhydria
Acholorhydria Patient counselling for peptic ulcer disease
Patient counselling for peptic ulcer disease Turburculousis
Turburculousis Gastric ulcer vs duodenal ulcer
Gastric ulcer vs duodenal ulcer What is the role of gastric juice
What is the role of gastric juice Morphology of peptic ulcer
Morphology of peptic ulcer Peptic ulcer classification
Peptic ulcer classification Peptic ulcer case study
Peptic ulcer case study Triple therapy for peptic ulcer disease
Triple therapy for peptic ulcer disease Asam national practice guideline
Asam national practice guideline Formal multiplication rule
Formal multiplication rule Ccgi guidelines
Ccgi guidelines Anamnesa psikologi adalah
Anamnesa psikologi adalah Cushing syndrome mnemonic
Cushing syndrome mnemonic Disconnected epidural catheter guideline
Disconnected epidural catheter guideline What is a guideline for hoisting a hoseline?
What is a guideline for hoisting a hoseline? Guideline anamnesa
Guideline anamnesa Petronas sus portal guideline
Petronas sus portal guideline Acg pancreatitis
Acg pancreatitis East guideline
East guideline Waikato stormwater management guideline
Waikato stormwater management guideline Escardio
Escardio Who guideline on country pharmaceutical pricing policies
Who guideline on country pharmaceutical pricing policies The turnbull guidelines
The turnbull guidelines Patient safety incident reporting form
Patient safety incident reporting form Aki kdigo 2012
Aki kdigo 2012 Bpfk cosmetic guideline
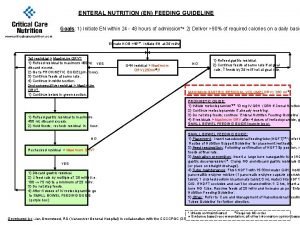
Bpfk cosmetic guideline En feeding guide
En feeding guide Interview guideline template
Interview guideline template Asean stability guideline
Asean stability guideline 5 guideline for cumbersome calculations
5 guideline for cumbersome calculations Outside counsel guidelines template
Outside counsel guidelines template Msqh standard
Msqh standard Mfmea example
Mfmea example Leischker
Leischker Hair cutting angles
Hair cutting angles Anemia in pregnancy guideline
Anemia in pregnancy guideline Halimbawa ng bahagi ng pahayagan
Halimbawa ng bahagi ng pahayagan Korea nazarene university
Korea nazarene university Summary of cold war
Summary of cold war Werner sasse
Werner sasse Sat subject2 korean
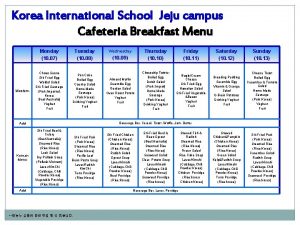
Sat subject2 korean Cafeteria
Cafeteria Korean western power
Korean western power