1516 Semester genap Corrosion process and control TKK2289




- Slides: 20

15/16 Semester genap Corrosion process and control (TKK-2289) Instructor: Rama Oktavian; Vivi Nurhadianty. Email: rama. oktavian 86@gmail. com Office Hr. : T. 11 -12, Th. 08 -10; 13 -15, F. 08 -10; 13 -15

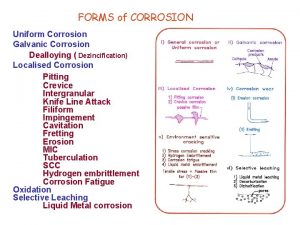
Corrosion types Hydrogen damage - embrittlement is not the only way in which materials are damaged by hydrogen. Steels are also damaged by hydrogen blistering at high temperatures - three categories of hydrogen damage: a. High temperature hydrogen attack b. Hydrogen blistering c. Hydrogen embrittlement

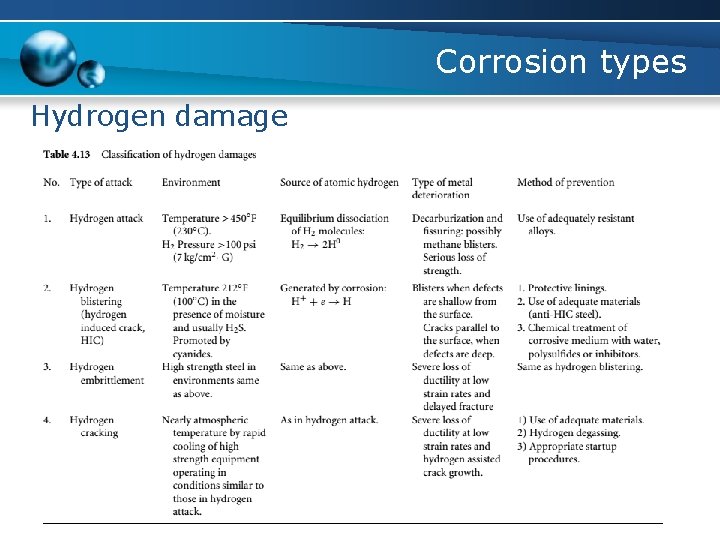
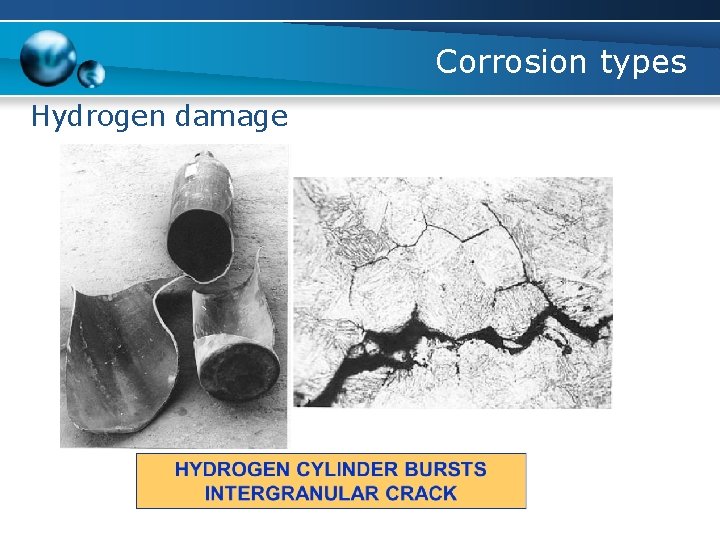
Corrosion types Hydrogen damage

Corrosion types Hydrogen damage

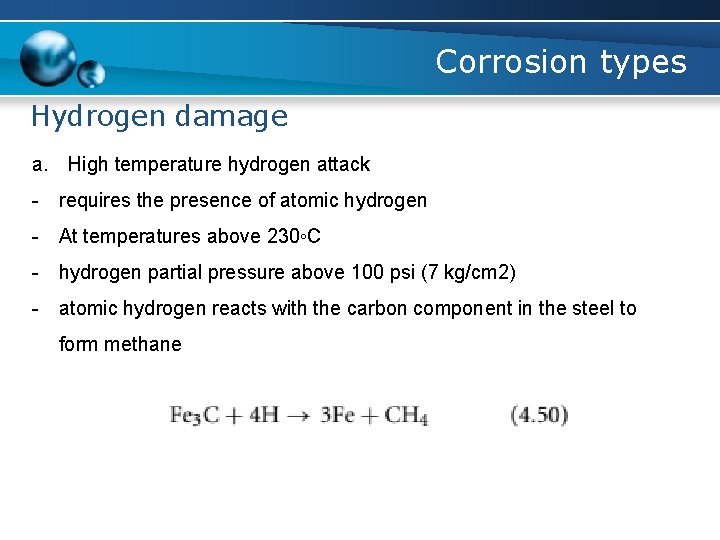
Corrosion types Hydrogen damage a. High temperature hydrogen attack - requires the presence of atomic hydrogen - At temperatures above 230◦C - hydrogen partial pressure above 100 psi (7 kg/cm 2) - atomic hydrogen reacts with the carbon component in the steel to form methane

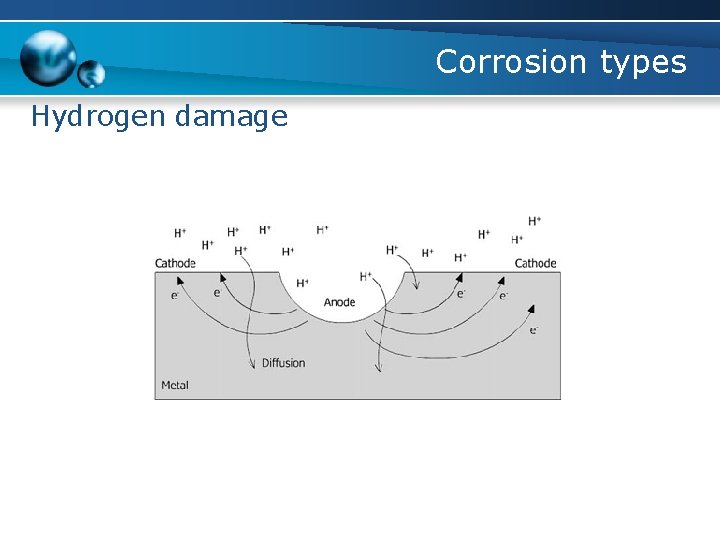
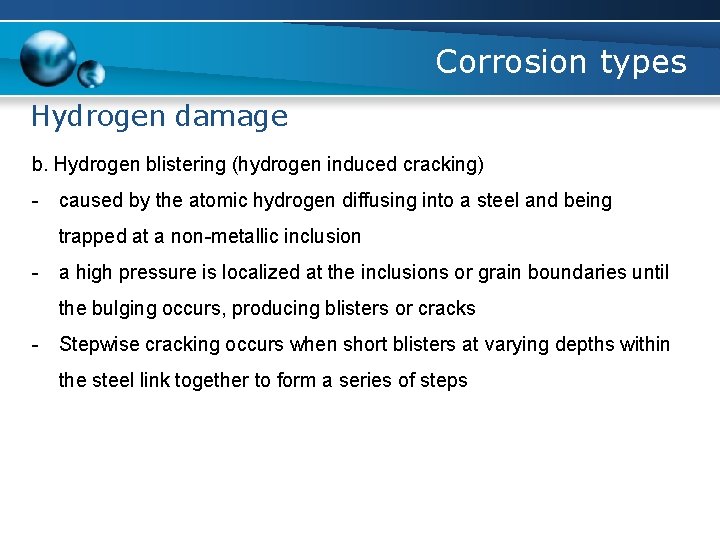
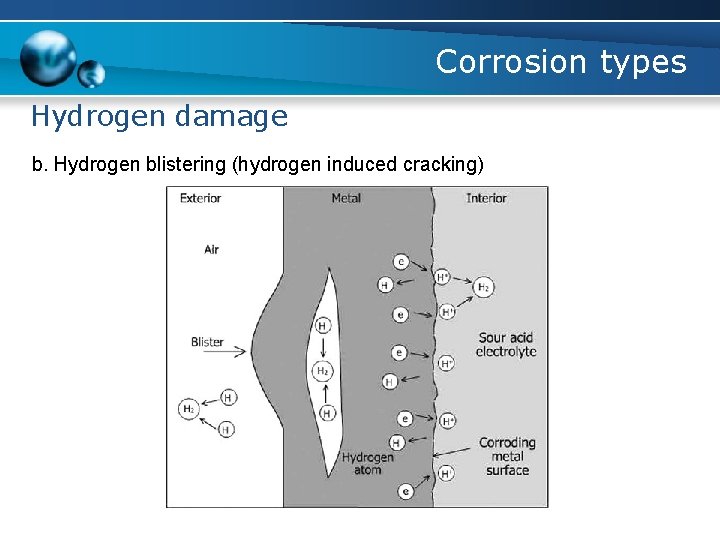
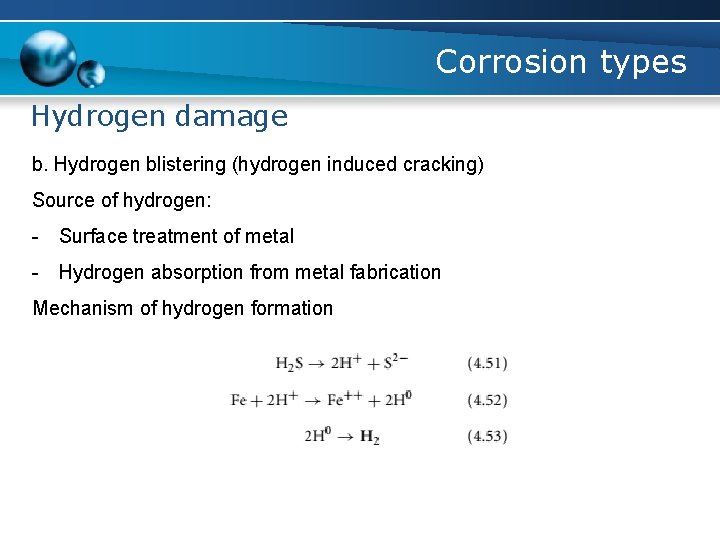
Corrosion types Hydrogen damage b. Hydrogen blistering (hydrogen induced cracking) - caused by the atomic hydrogen diffusing into a steel and being trapped at a non-metallic inclusion - a high pressure is localized at the inclusions or grain boundaries until the bulging occurs, producing blisters or cracks - Stepwise cracking occurs when short blisters at varying depths within the steel link together to form a series of steps

Corrosion types Hydrogen damage b. Hydrogen blistering (hydrogen induced cracking)

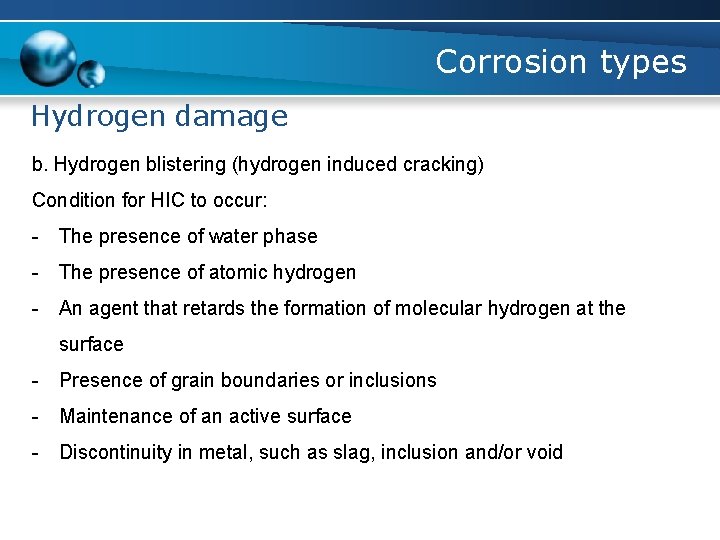
Corrosion types Hydrogen damage b. Hydrogen blistering (hydrogen induced cracking) Condition for HIC to occur: - The presence of water phase - The presence of atomic hydrogen - An agent that retards the formation of molecular hydrogen at the surface - Presence of grain boundaries or inclusions - Maintenance of an active surface - Discontinuity in metal, such as slag, inclusion and/or void

Corrosion types Hydrogen damage b. Hydrogen blistering (hydrogen induced cracking) Source of hydrogen: - Surface treatment of metal - Hydrogen absorption from metal fabrication Mechanism of hydrogen formation

Corrosion types Hydrogen damage c. Hydrogen embrittlement - Cathodic hydrogen is adsorbed on the surface as atomic hydrogen (reduced) - The internal pressure produced by the gaseous hydrogen is much lower than produced by cathodic hydrogen - occurs during the plastic deformation of alloys in contact with hydrogen gas

Corrosion types Hydrogen damage c. Hydrogen embrittlement Example: - In plating operations. - In pickling operations - In cleaning of high strength steels in chloride or fluoride solution - Manufacturing and fabrication processes Materials that are most susceptible to hydrogen embrittlement: Iron, titanium, zirconium, martensitic steels, high strength aluminum alloys.

Corrosion types Hydrogen damage c. Hydrogen embrittlement Example: - In plating operations. - In pickling operations - In cleaning of high strength steels in chloride or fluoride solution - Manufacturing and fabrication processes Materials that are most susceptible to hydrogen embrittlement: Iron, titanium, zirconium, martensitic steels, high strength aluminum alloys.

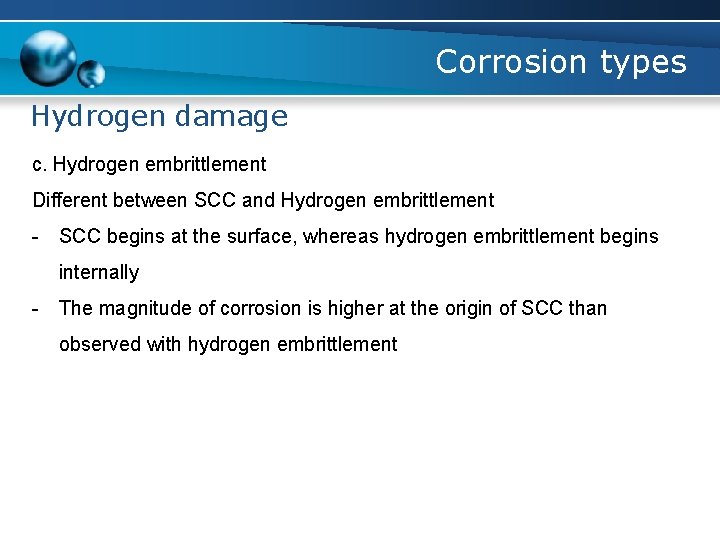
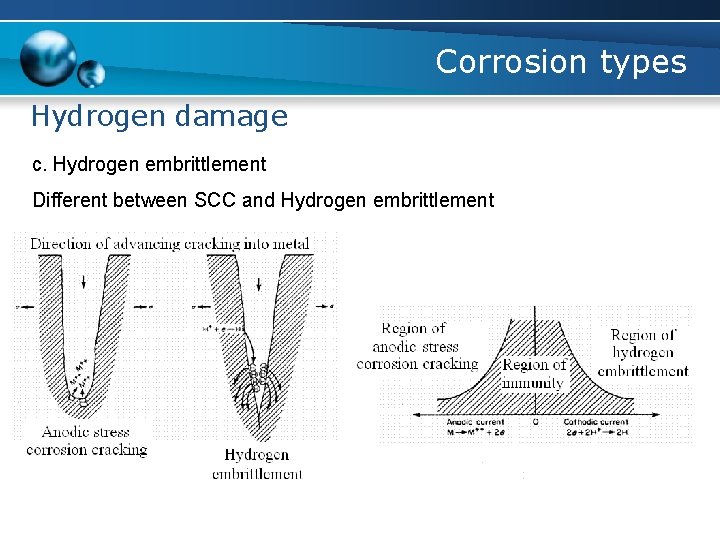
Corrosion types Hydrogen damage c. Hydrogen embrittlement Different between SCC and Hydrogen embrittlement - SCC begins at the surface, whereas hydrogen embrittlement begins internally - The magnitude of corrosion is higher at the origin of SCC than observed with hydrogen embrittlement

Corrosion types Hydrogen damage c. Hydrogen embrittlement Different between SCC and Hydrogen embrittlement

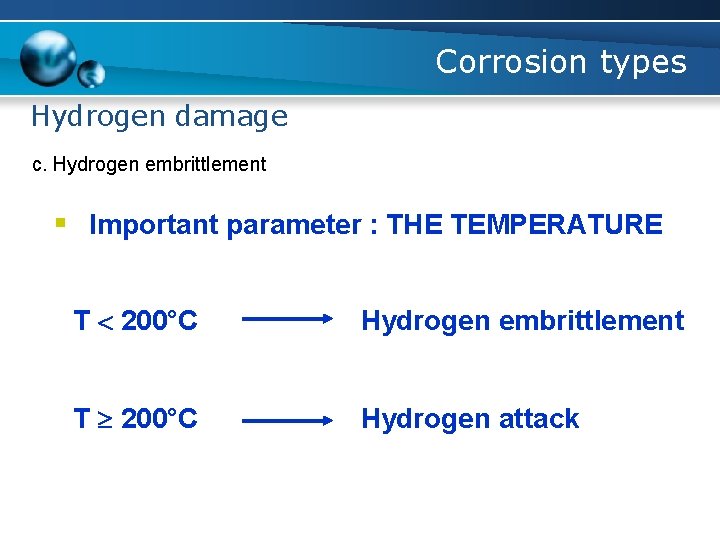
Corrosion types Hydrogen damage c. Hydrogen embrittlement § Important parameter : THE TEMPERATURE T 200°C Hydrogen embrittlement T 200°C Hydrogen attack

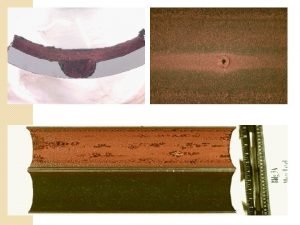
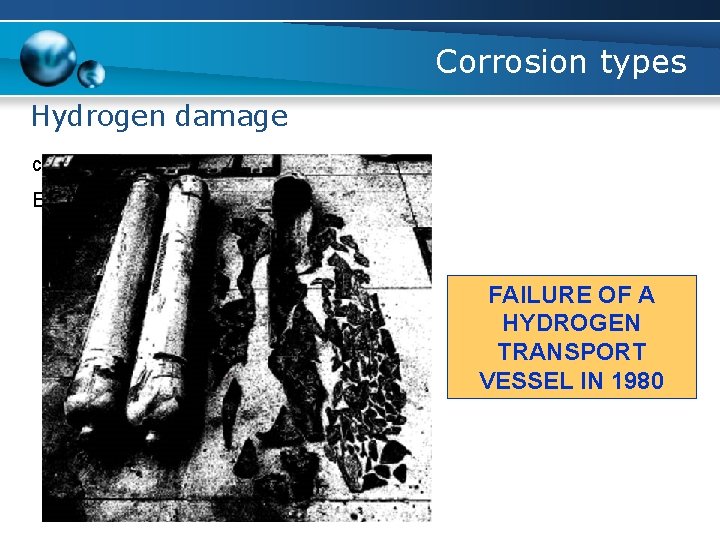
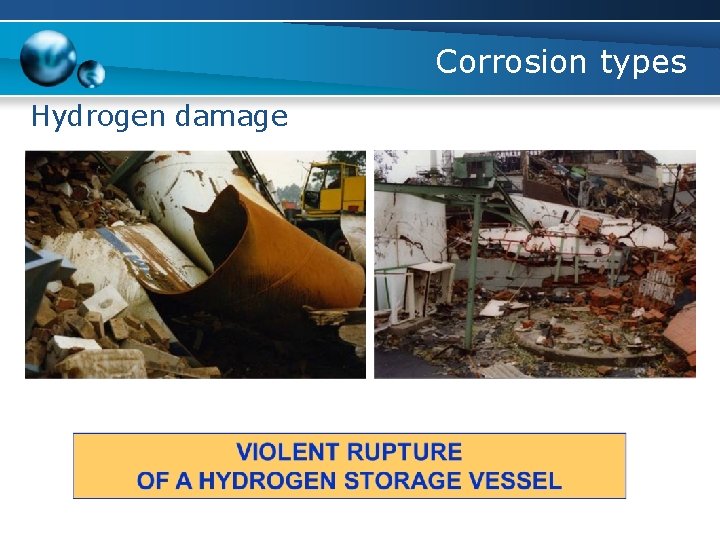
Corrosion types Hydrogen damage c. Hydrogen embrittlement Example FAILURE OF A HYDROGEN TRANSPORT VESSEL IN 1980

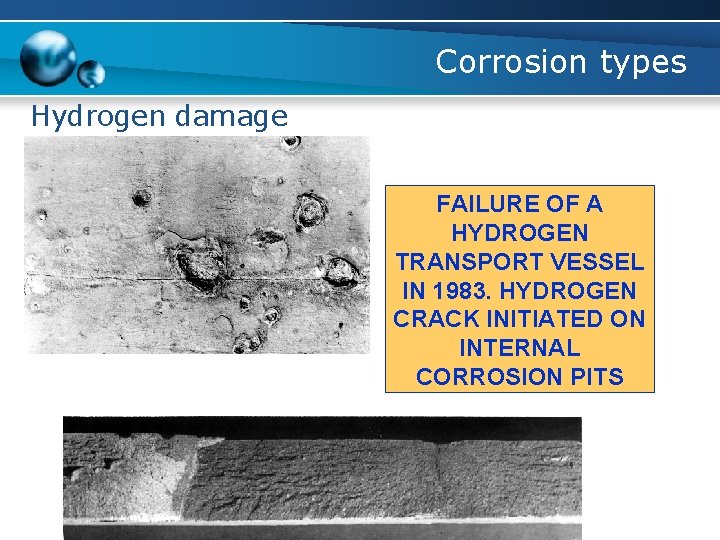
Corrosion types Hydrogen damage FAILURE OF A HYDROGEN TRANSPORT VESSEL IN 1983. HYDROGEN CRACK INITIATED ON INTERNAL CORROSION PITS

Corrosion types Hydrogen damage

Corrosion types Hydrogen damage

 Differentiate between dry corrosion and wet corrosion
Differentiate between dry corrosion and wet corrosion Classification of corrosion
Classification of corrosion Materi akidah akhlak kelas 7 semester genap
Materi akidah akhlak kelas 7 semester genap Corrosion control solutions
Corrosion control solutions Product control
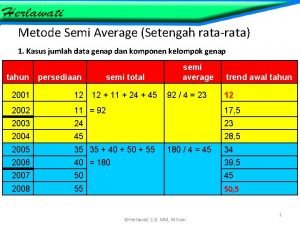
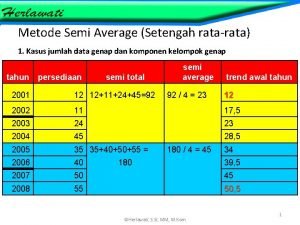
Product control Metode semi average
Metode semi average Contoh soal metode semi average data ganjil
Contoh soal metode semi average data ganjil Deret fourier fungsi genap
Deret fourier fungsi genap Contoh metode least square
Contoh metode least square Trend setengah rata-rata
Trend setengah rata-rata Deret fourier
Deret fourier Diantara bilangan bulat antara 101 - 600
Diantara bilangan bulat antara 101 - 600 Daun tunggal adalah
Daun tunggal adalah Rumus median ganjil
Rumus median ganjil Nombor genap in english
Nombor genap in english Difference between dry and wet corrosion
Difference between dry and wet corrosion Uniform corrosion
Uniform corrosion Corrosion prevention casing filler
Corrosion prevention casing filler Corrosion under insulation primer
Corrosion under insulation primer Aluminium corrosion equation
Aluminium corrosion equation Skin corrosion symbol
Skin corrosion symbol