1 2 Terms and Configurations Transition Metals Terms




- Slides: 23

1. 2 Terms and Configurations Transition Metals

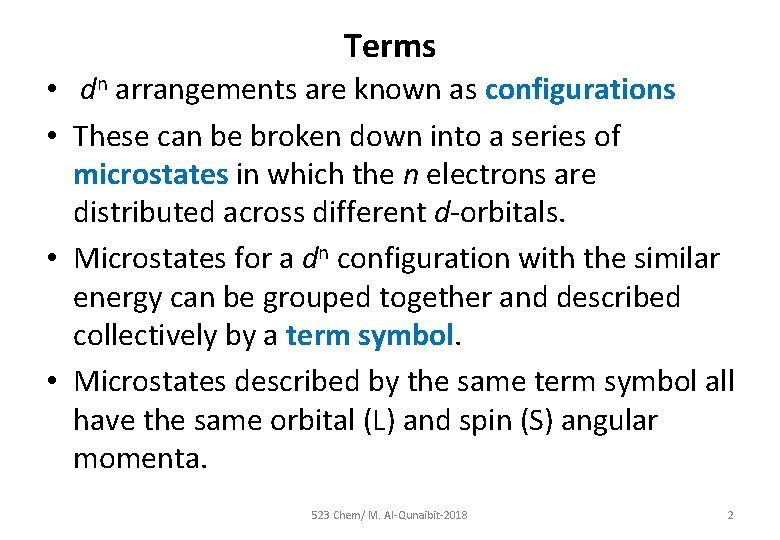
Terms • dn arrangements are known as configurations • These can be broken down into a series of microstates in which the n electrons are distributed across different d-orbitals. • Microstates for a dn configuration with the similar energy can be grouped together and described collectively by a term symbol. • Microstates described by the same term symbol all have the same orbital (L) and spin (S) angular momenta. 523 Chem/ M. Al-Qunaibit-2018 2

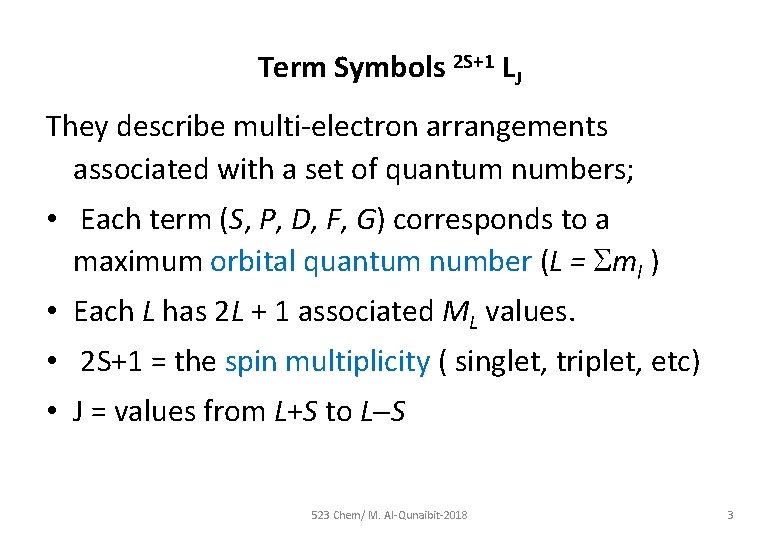
Term Symbols 2 S+1 LJ They describe multi-electron arrangements associated with a set of quantum numbers; • Each term (S, P, D, F, G) corresponds to a maximum orbital quantum number (L = ml ) • Each L has 2 L + 1 associated ML values. • 2 S+1 = the spin multiplicity ( singlet, triplet, etc) • J = values from L+S to L S 523 Chem/ M. Al-Qunaibit-2018 3

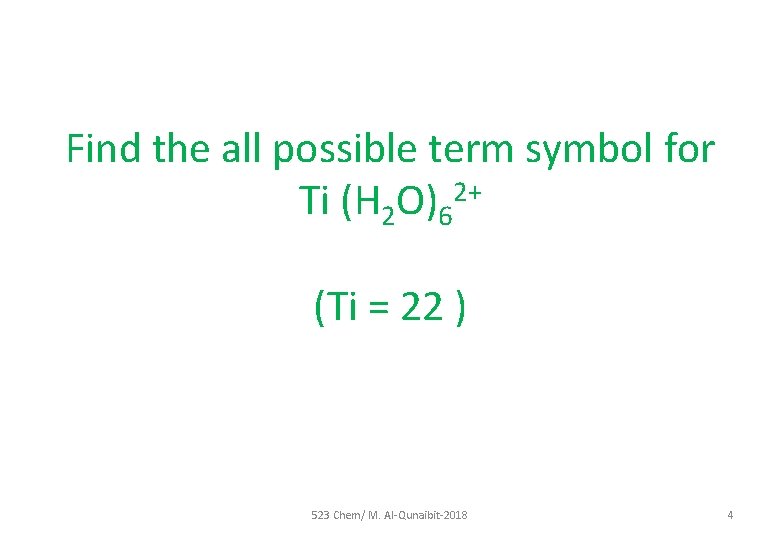
Find the all possible term symbol for Ti (H 2 O)62+ (Ti = 22 ) 523 Chem/ M. Al-Qunaibit-2018 4

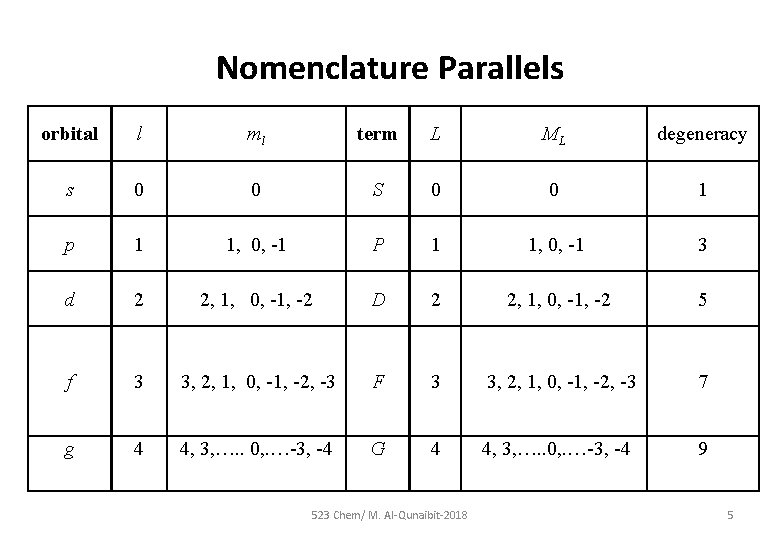
Nomenclature Parallels orbital l ml term L ML degeneracy s 0 0 S 0 0 1 p 1 1, 0, -1 P 1 1, 0, -1 3 d 2 2, 1, 0, -1, -2 D 2 2, 1, 0, -1, -2 5 f 3 3, 2, 1, 0, -1, -2, -3 F 3 3, 2, 1, 0, -1, -2, -3 7 g 4 4, 3, …. . 0, . …-3, -4 G 4 4, 3, …. . 0, . …-3, -4 9 523 Chem/ M. Al-Qunaibit-2018 5

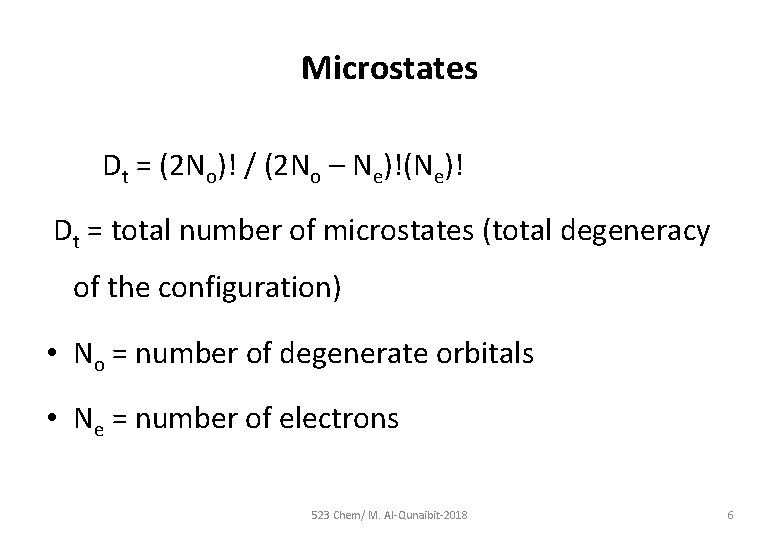
Microstates Dt = (2 No)! / (2 No – Ne)!(Ne)! Dt = total number of microstates (total degeneracy of the configuration) • No = number of degenerate orbitals • Ne = number of electrons 523 Chem/ M. Al-Qunaibit-2018 6

Find the number of microstates for 2+ Ti (Ti = 22 ) 523 Chem/ M. Al-Qunaibit-2018 7

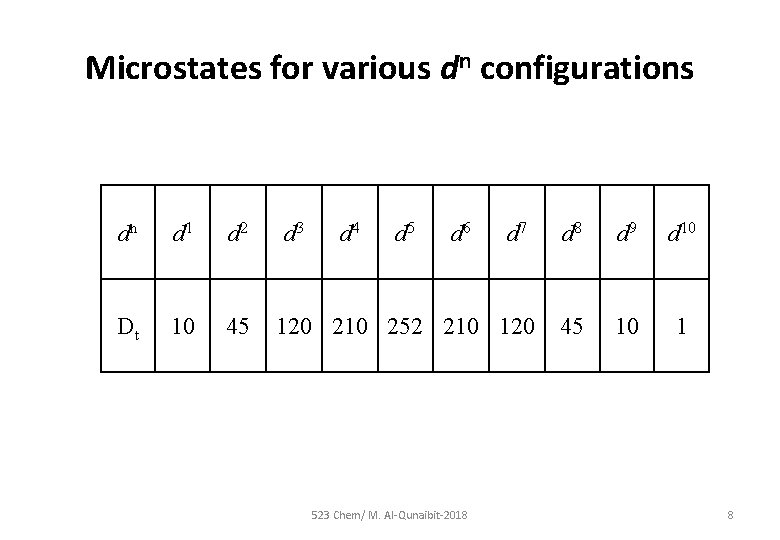
Microstates for various dn configurations dn d 1 d 2 Dt 10 45 d 3 d 4 d 5 d 6 d 7 120 210 252 210 120 523 Chem/ M. Al-Qunaibit-2018 d 9 d 10 45 10 1 8

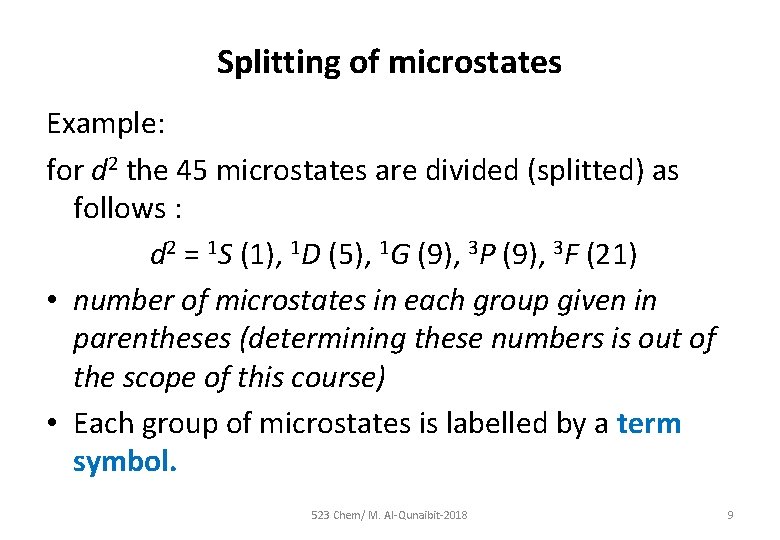
Splitting of microstates Example: for d 2 the 45 microstates are divided (splitted) as follows : d 2 = 1 S (1), 1 D (5), 1 G (9), 3 P (9), 3 F (21) • number of microstates in each group given in parentheses (determining these numbers is out of the scope of this course) • Each group of microstates is labelled by a term symbol. 523 Chem/ M. Al-Qunaibit-2018 9

Energy of Microstates • Not all microstates of a dn configuration are equal in energy. • The microstates divide into several groups, in which each group differs in energy from the others, but within each group all the microstates have the same orbital (L) and spin (S) angular momenta. 523 Chem/ M. Al-Qunaibit-2018 10

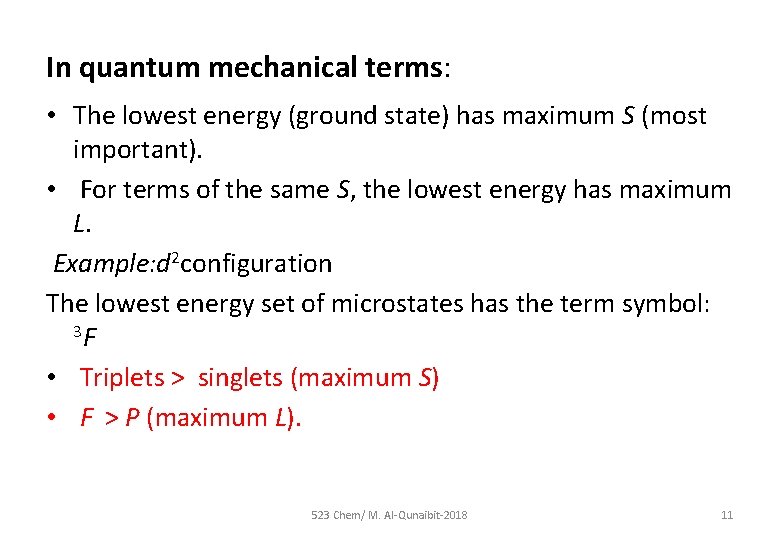
In quantum mechanical terms: • The lowest energy (ground state) has maximum S (most important). • For terms of the same S, the lowest energy has maximum L. Example: d 2 configuration The lowest energy set of microstates has the term symbol: 3 F • Triplets > singlets (maximum S) • F > P (maximum L). 523 Chem/ M. Al-Qunaibit-2018 11

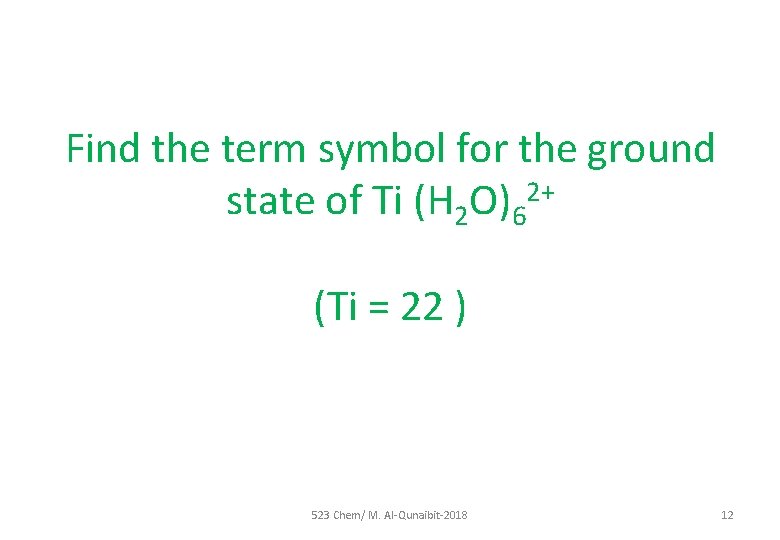
Find the term symbol for the ground state of Ti (H 2 O)62+ (Ti = 22 ) 523 Chem/ M. Al-Qunaibit-2018 12

Put the following terms in ascending sequence of energy 1 S, 1 D, 3 P, 1 G, 3 F 523 Chem/ M. Al-Qunaibit-2018 13

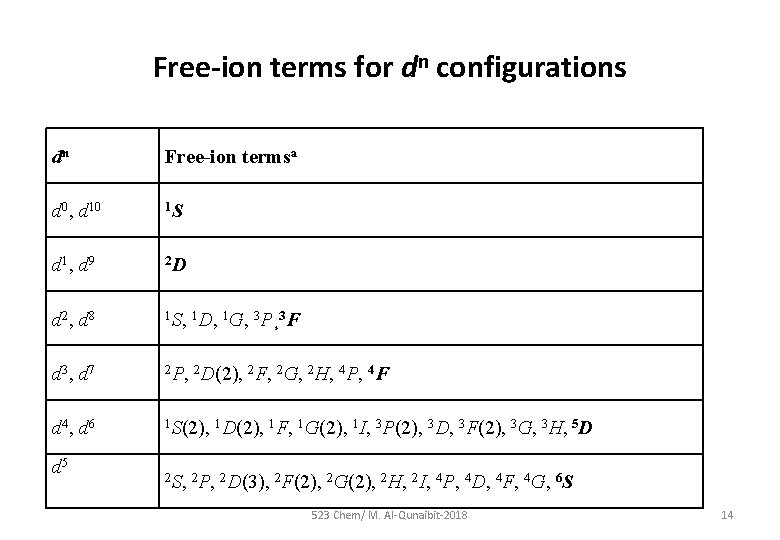
Free-ion terms for dn configurations dn Free-ion termsa d 0, d 10 1 S d 1, d 9 2 D d 2, d 8 1 S, 1 D, 1 G, 3 P¸ 3 F d 3, d 7 2 P, 2 D(2), 2 F, 2 G, 2 H, 4 P, 4 F d 4, d 6 1 S(2), 1 D(2), 1 F, 1 G(2), 1 I, 3 P(2), 3 D, 3 F(2), 3 G, 3 H, 5 D d 5 2 S, 2 P, 2 D(3), 2 F(2), 2 G(2), 2 H, 2 I, 4 P, 4 D, 4 F, 4 G, 6 S 523 Chem/ M. Al-Qunaibit-2018 14

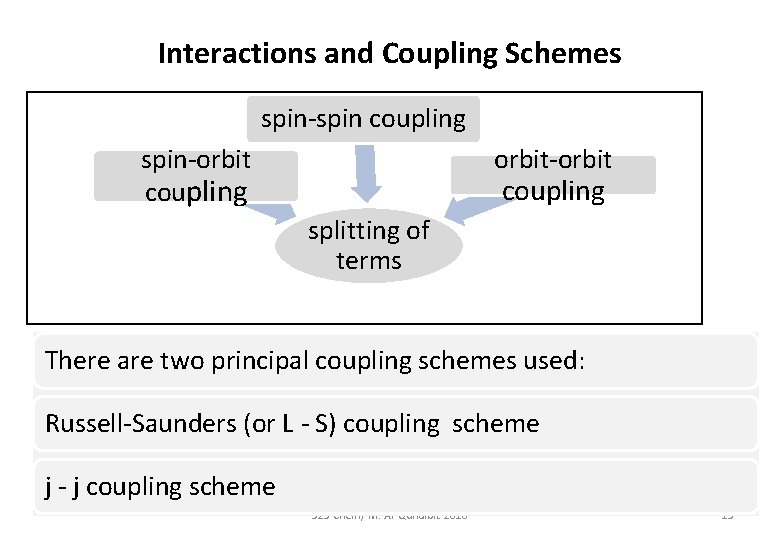
Interactions and Coupling Schemes spin-spin coupling orbit-orbit spin-orbit coupling splitting of terms There are two principal coupling schemes used: Russell-Saunders (or L - S) coupling scheme j - j coupling scheme 523 Chem/ M. Al-Qunaibit-2018 15

The Russell Saunders Coupling Scheme • It is assumed that: spin-spin coupling > orbit-orbit coupling > spinorbit coupling. • This is found to give a good approximation for first row transition series where J coupling is ignored. • For elements with atomic numbers greater than 30, spin-orbit coupling becomes more significant and the j-j coupling scheme is used. 523 Chem/ M. Al-Qunaibit-2018 16

j-j coupling scheme For heavier elements with larger nuclear charge: • The spin-orbit interactions become as strong as the interactions between individual spins or orbital angular momenta. • In those cases the spin and orbital angular momenta of individual electrons tend to couple to form individual electron angular momenta. 523 Chem/ M. Al-Qunaibit-2018 17

Find the ground term for each of the following d 2 ions): Ti 2+ Zr 2+ 523 Chem/ M. Al-Qunaibit-2018 18

Symmetry Labels for dn Configurations For octahedral complexes with d 4 – d 7 configurations there are two possible lowest energy situations (ground state) depending on: • the relative magnitudes of o (the ligand field energy) • and the pairing energy 523 Chem/ M. Al-Qunaibit-2018 19

High Spin vs. Low Spin complexes For Oh complexes: • Low-spin complexes occur in strong ligand fields • High-spin complexes arise when o is small, a weak ligand field For Td complexes: • t is small and always equates to a weak field; tetrahedral complexes are always high-spin. 523 Chem/ M. Al-Qunaibit-2018 20
What is the effect of each of strong and weak field ligands on d-electrons in transition metals? 523 Chem/ M. Al-Qunaibit-2018 21

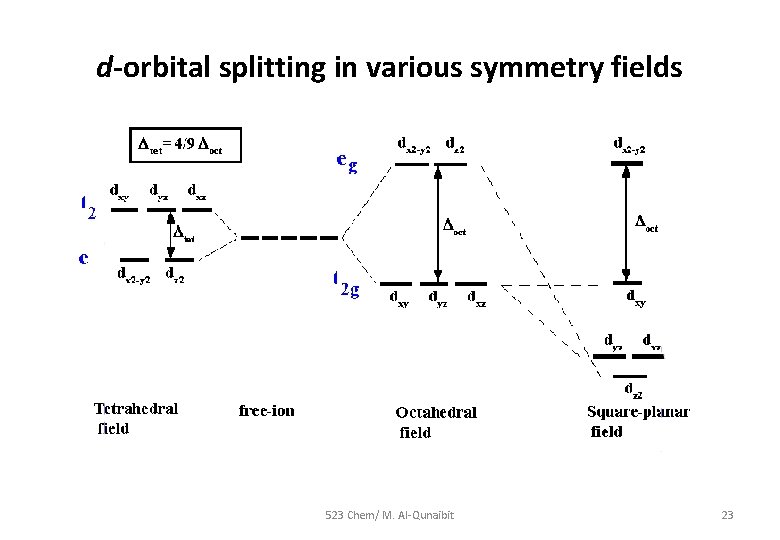
Symmetry labels under a ligand field effect • Based on the technique of direct products we can evaluate the symmetry label for any dn configuration for a complex of Oh symmetry (out of the scope of this course). • As a result, and just as d-orbitals split under a ligand field, so do terms; For Oh complexes: Eg and T 2 g For Td complexes : T 2 and E 523 Chem/ M. Al-Qunaibit 22

d-orbital splitting in various symmetry fields 523 Chem/ M. Al-Qunaibit 23