Weather and Water INVESTIGATION 3 AIR PRESSURE AND



















- Slides: 31

Weather and Water INVESTIGATION 3: AIR PRESSURE AND WIND FOCUS QUESTION 3. 1: HOW DOES PRESSURE AFFECT AIR?

When you see the following symbol please be prepared to write or place something into your notebook!!

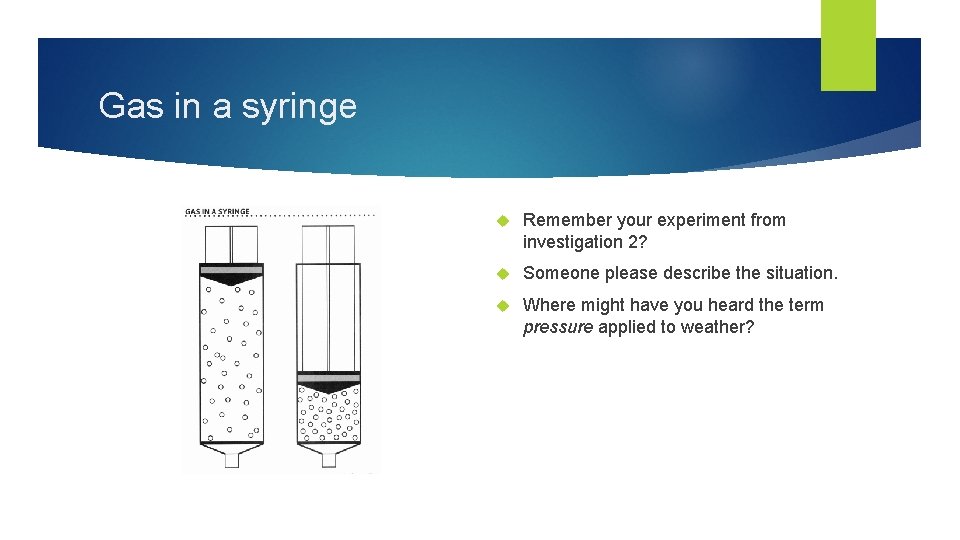
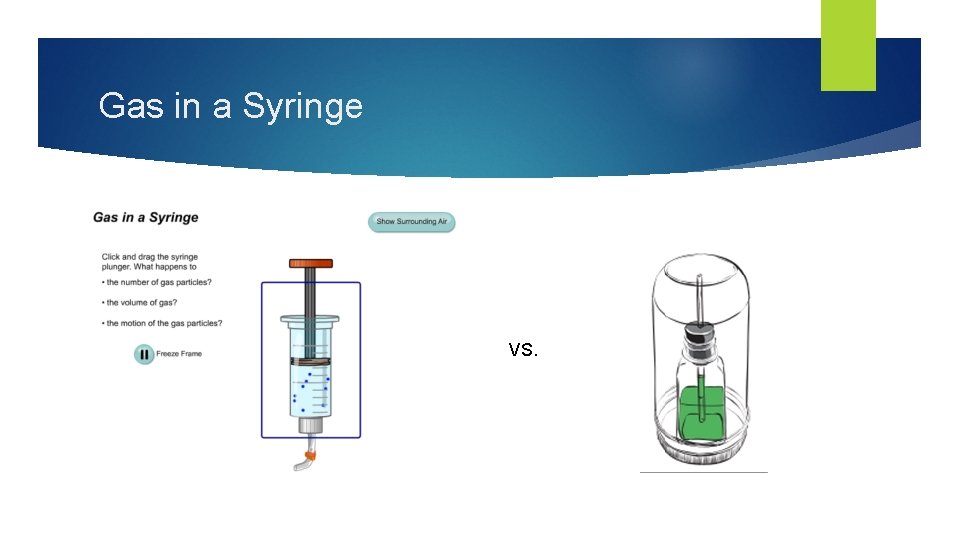
Gas in a syringe Remember your experiment from investigation 2? Someone please describe the situation. Where might have you heard the term pressure applied to weather?

Focus Question How does pressure affect air?

Inquiry Introduction

Setting up our inquiry Getter 1 Go Get some GREEN Water at the sink. You will need to take your square bottle.

Part 2: Explore the jar After you have assembled your pressure indicator, begin answering the question in Part 2. Don’t forget to draw your diagrams.

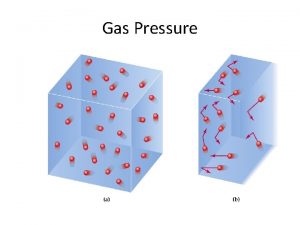
Let’s Discuss: Any surprises? Is the volume inside the plastic jar greater, the same, or less when you squeeze it? Think about the particles of air in the jar. Can they be pushed closer together? How does squeezing the jar push the particles together? Think back to the syringe online activity, and the way the air particles moved when compressed. If you could see the air particles in the bottle, what would you see near the surface of the water? Less Yes The same number of particles are forced into a smaller space. The air particles would be bumping into the surface of the water more, as they did in the syringe, because they are pushed closer together and colliding against each other.

Discuss with your group. Use your diagrams and the vocabulary on the vocab wall to facilitate your discussion. Why does the water in the tube go down when you push on the sides of the plastic jar? When we squeeze the jar, the volume in the jar is less. The air is compressed. The particles are closer together, so they bump into each other and the sides of the jar more often. This increases pressure inside the jar. The air inside the top of the clear tube is also compressed. It pushes down with more pressure on the top of the water in the tube. Water doesn’t compress, but it puts pressure on the air inside the glass bottle. When the air inside the jar is compressed, it puts pressure on the water in the tube and jar, which puts pressure on the air inside the little glass bottle, which compresses the air in the bottle. The compressed air goes into a smaller space. Water moves down the tube to fill the space that used to be filled with air. That’s why the water level in the straw goes down when pressure is applied.

Everybody Clean Up Time Disassemble the pressure-indicator systems. Getter 2, pour the green water into the sink and dry the best you can. Everyone else clean up your area and leave it the way it was when you came in. Finally, reflect on our focus question; How does air pressure affect air? We will answer this later! GREAT JOB EVERYONE!!

Weather and Water INVESTIGATION 3: AIR PRESSURE AND WIND FOCUS QUESTION 3. 1: HOW DOES PRESSURE AFFECT AIR?

Gas in a Syringe VS.

Something happened as we added pressure to the syringe and to the pressure-indicator system It decreased. What happened to the volume of the jar surrounding the glass bottle? What happened to the number of particles It stayed the same. in the jar? If the number of particles stayed the same, It stayed the same. what happened to the total mass of the particles?

Gas in a syringe When particles get pushed closer together because of pressure, the particles get pushed into a smaller space. The air increases in density Density refers to the mass of materials in a given space. Of the two syringes, which has air that is more dense?

Weather Connections Remember to think of air pressure as air particles hitting things. What will happen if a syringe full of compressed air is suddenly opened? Why will air leave the syringe? The air will rush out. The outside air is at lower pressure, and the particles will move out until the pressure is equal/ When air moves from an area of high pressure to one of low pressure, it is returning to equilibrium. Think of the word equal when you think of equilibrium. If air is under high pressure in one area and has less space to move around in, if it is able to escape, air particles will flow from the area of high pressure until the pressure, or air density, is equal. In nature, if an area of higher air pressure occurs in real life, how will it reach equilibrium?

Let’s Read “What is Air Pressure? ” in your textbook pages 29 -33. Vocabulary Kinetic energy Atmospheric pressure Define: Barometer Define: Big Idea When a volume of air is compressed, particles are closer together, and the air become more dense.

Barometer These are some examples of physical barometers. We’ve been using one with digital readout, but they collect data about atmospheric pressure in a similar way. The prefix baro- means pressure, and the suffix –meter means measure. In meteorology, air pressure is measured in bars. A bar is equal to the pressure exerted by the air in the atmosphere at sea level. The unit usually used to measure atmospheric pressure is millibar (mb), equal to 1/1000 of a bar. Average sea-level air pressure is 1013 mb.

“Barometer in a Bottle” Opening Screen What could we do to change the pressure reading on the barometer in the jar? Watch video. Keep track of barometer readings. Why do you think the pressure reading changed? The particles were squeezed into a smaller volume, and the air pressure increased. Increase the volume of the jar What could we do to decrease the airpressure reading?

Atmospheric Pressure FOSS WEB: Weather-Balloon Simulation FOSS WEB: Elevator to Space

Let’s try to answer our focus question now. How does pressure affect air?

Weather and Water INVESTIGATION 3: AIR PRESSURE AND WIND FOCUS QUESTION 3. 2: WHAT HAPPENS WHEN TWO AREAS OF AIR HAVE DIFFERENT PRESSURES?

Weather Map Introductions In the last part of this investigation, we talked about equilibrium. If air in a container is under higher pressure than the surrounding air, when the container is opened, air will flow out until there is equilibrium, so all the air is at the same density and same pressure. What happens in nature when air can flow freely, and is not held in containers like syringes? What happens when two areas of air have different pressures?

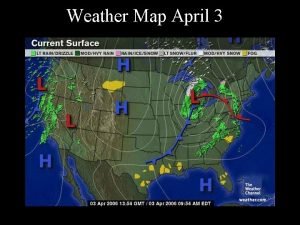
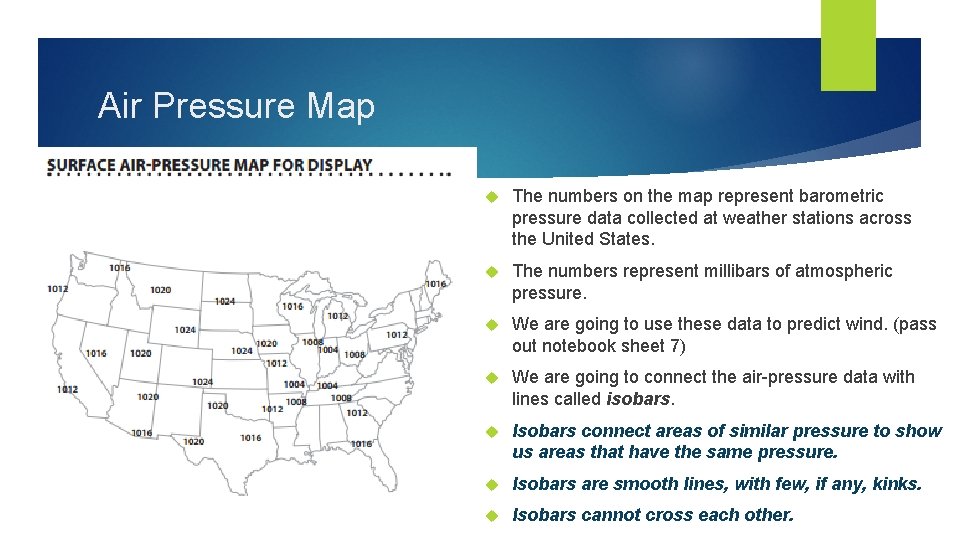
Air Pressure Map The numbers on the map represent barometric pressure data collected at weather stations across the United States. The numbers represent millibars of atmospheric pressure. We are going to use these data to predict wind. (pass out notebook sheet 7) We are going to connect the air-pressure data with lines called isobars. Isobars connect areas of similar pressure to show us areas that have the same pressure. Isobars are smooth lines, with few, if any, kinks. Isobars cannot cross each other.

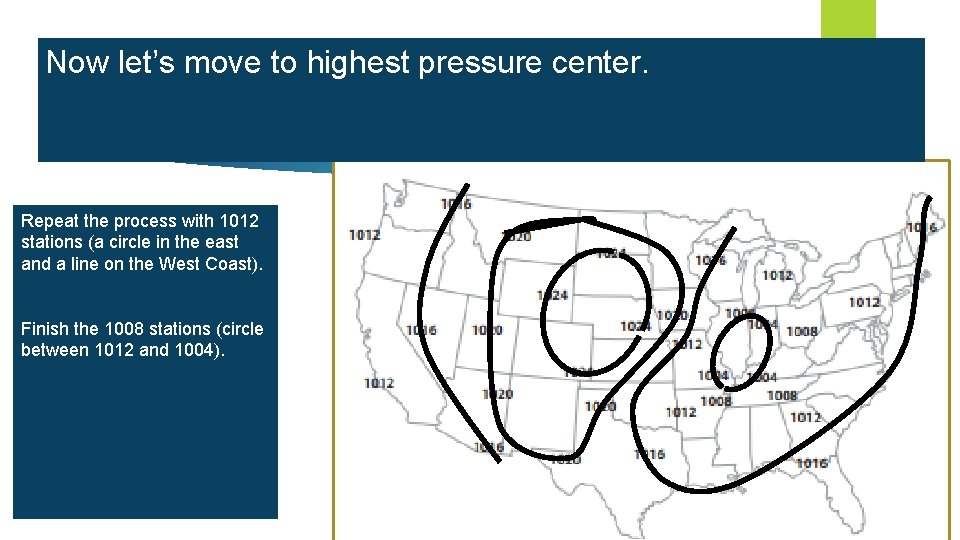
Now let’s move to highest pressure center. Let’s begin with the air lowest pressure. Which stations have the lowest millibars pressure? Isobars areas of What Repeat is theconnect process next lowest withreading 1012 similar us stations from a weather (apressure circlestation? in to theshow east 1020 the same andareas a linethat on have the West Coast). pressure. Isobars are smooth Now let’s do 1016. lines, Finish 1008 stations withthe few, if any, kinks. (circle between 1004). This can be 1012 tricky. and Remember we can’t Isobars cannot cross each cross other isobar lines, and the 1020 other. the map into east and line separates west. Lets connect the 1016 stations in Plan how you are going to the east together into a circle or loop. your before The draw western 1016 line stations we can starting. connect with a separate line, because we can’t cross other isobar lines. DO NOT LIFT YOUR PENCIL!!

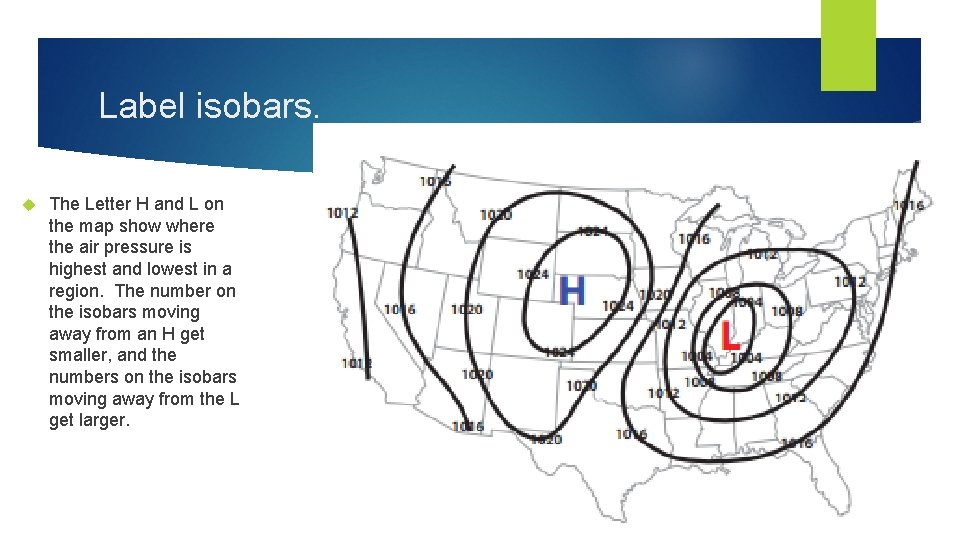
Label isobars. The Letter H and L on the map show where the air pressure is highest and lowest in a region. The number on the isobars moving away from an H get smaller, and the numbers on the isobars moving away from the L get larger.

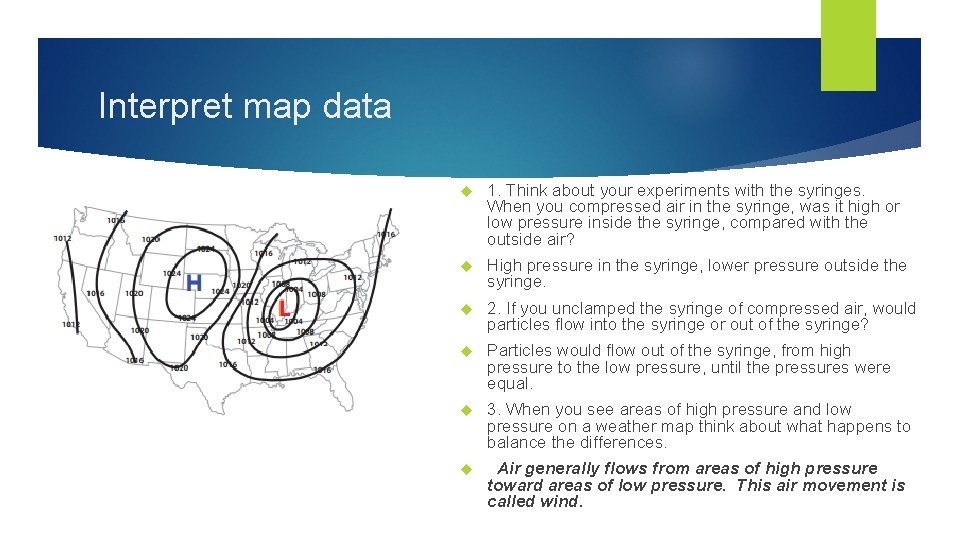
Interpret map data 1. Think about your experiments with the syringes. When you compressed air in the syringe, was it high or low pressure inside the syringe, compared with the outside air? High pressure in the syringe, lower pressure outside the syringe. 2. If you unclamped the syringe of compressed air, would particles flow into the syringe or out of the syringe? Particles would flow out of the syringe, from high pressure to the low pressure, until the pressures were equal. 3. When you see areas of high pressure and low pressure on a weather map think about what happens to balance the differences. Air generally flows from areas of high pressure toward areas of low pressure. This air movement is called wind.

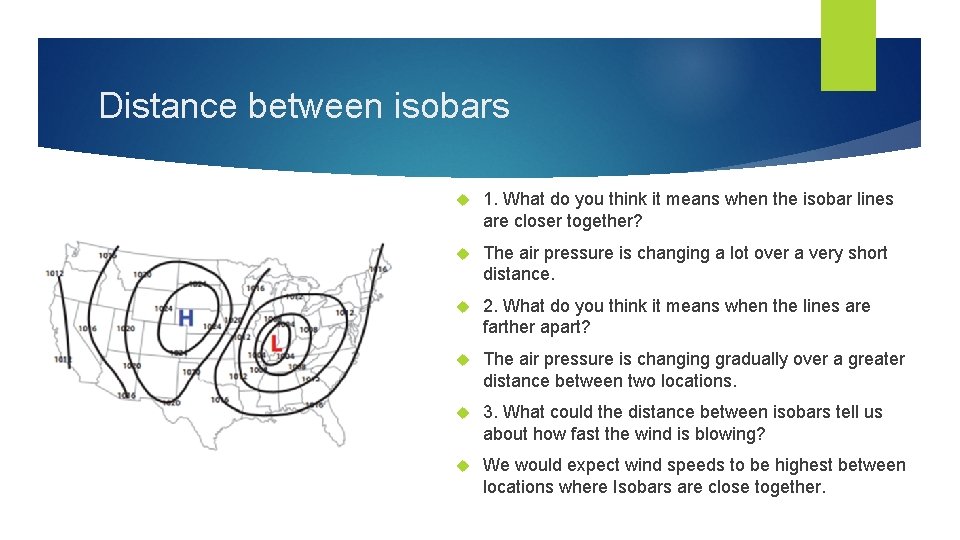
Distance between isobars 1. What do you think it means when the isobar lines are closer together? The air pressure is changing a lot over a very short distance. 2. What do you think it means when the lines are farther apart? The air pressure is changing gradually over a greater distance between two locations. 3. What could the distance between isobars tell us about how fast the wind is blowing? We would expect wind speeds to be highest between locations where Isobars are close together.

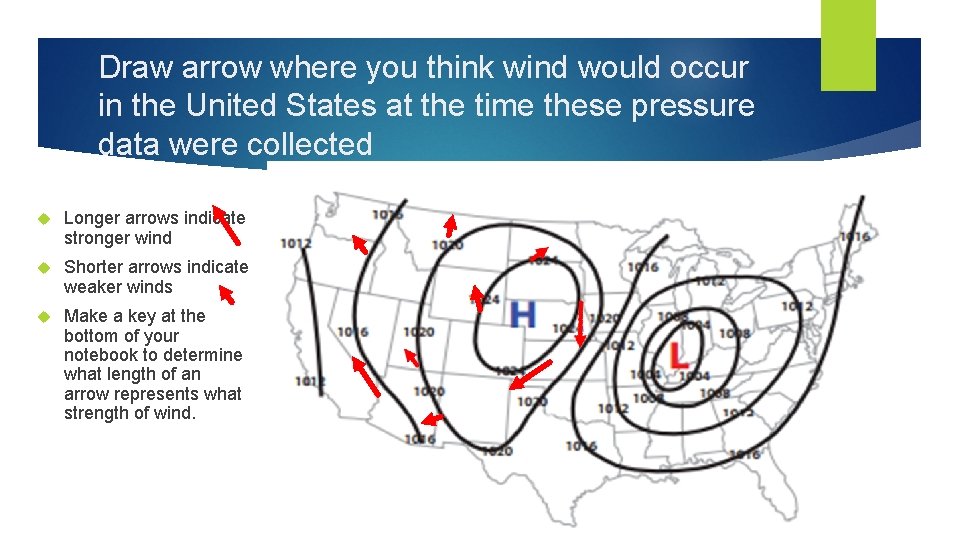
Draw arrow where you think wind would occur in the United States at the time these pressure data were collected Longer arrows indicate stronger wind Shorter arrows indicate weaker winds Make a key at the bottom of your notebook to determine what length of an arrow represents what strength of wind.

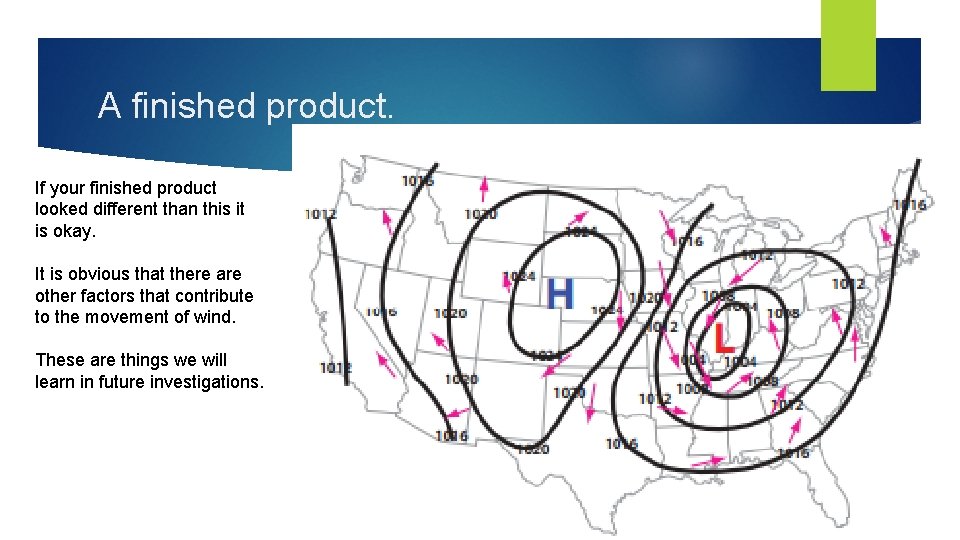
A finished product. If your finished product looked different than this it is okay. It is obvious that there are other factors that contribute to the movement of wind. These are things we will learn in future investigations.

BIG IDEA Air moves from areas of high pressure to low pressure.

Focus questions How does pressure affect air? What happens when two areas of air have the different pressure?
 Water and water and water water
Water and water and water water Why does low air pressure usually indicate stormy weather
Why does low air pressure usually indicate stormy weather An instrument used to measure temperature
An instrument used to measure temperature Air mass vocabulary
Air mass vocabulary Air higroskopis air kapiler dan air gravitasi
Air higroskopis air kapiler dan air gravitasi Weather investigation
Weather investigation What makes water hard
What makes water hard Static pressure and dynamic pressure
Static pressure and dynamic pressure What is oncotic pressure of blood
What is oncotic pressure of blood High pressure and low pressure
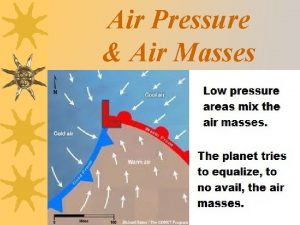
High pressure and low pressure Low atmospheric pressure
Low atmospheric pressure Weather station symbols
Weather station symbols Tongue twister about spring
Tongue twister about spring Her poem clothing
Her poem clothing Sunny rainy cloudy windy stormy
Sunny rainy cloudy windy stormy Weather vs whether
Weather vs whether Heavy weather by weather report
Heavy weather by weather report Capital weather gang weather wall
Capital weather gang weather wall Throchlea
Throchlea Pressure support vs pressure control
Pressure support vs pressure control Pressure mapping for pressure ulcers
Pressure mapping for pressure ulcers Intrapulmonary pressure vs intrapleural pressure
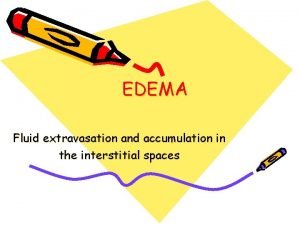
Intrapulmonary pressure vs intrapleural pressure Types of edema
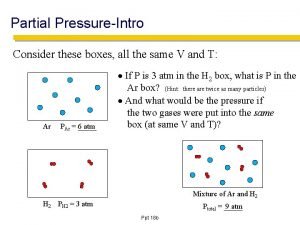
Types of edema Partial pressure
Partial pressure Tripod position breathing
Tripod position breathing How are metamorphic rocks classified
How are metamorphic rocks classified ütube
ütube Capillary filtration coefficient
Capillary filtration coefficient Colloid osmotic pressure vs hydrostatic pressure
Colloid osmotic pressure vs hydrostatic pressure Oncotic pressure vs hydrostatic pressure
Oncotic pressure vs hydrostatic pressure Confining pressure vs directed pressure
Confining pressure vs directed pressure Blood pressure regulation
Blood pressure regulation