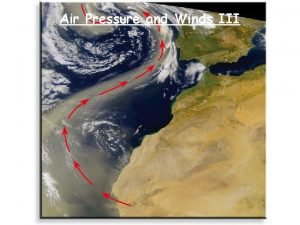
Air Pressure and Wind Air Pressure Inquiry Investigation















- Slides: 22

Air Pressure and Wind Air Pressure Inquiry Investigation 8, Part 1

Syringes and Pressure • Remember the activity with the syringes and tubing • Describe the investigation • The air was compressed when the plunger was squeezed • The blue foam cube became smaller because the air inside it was compressed

Air-Pressure Inquiry • Plastic jar that is tightly closed • The inside glass is half filled with water • A stopper with tubing is pushed tightly into the glass bottle

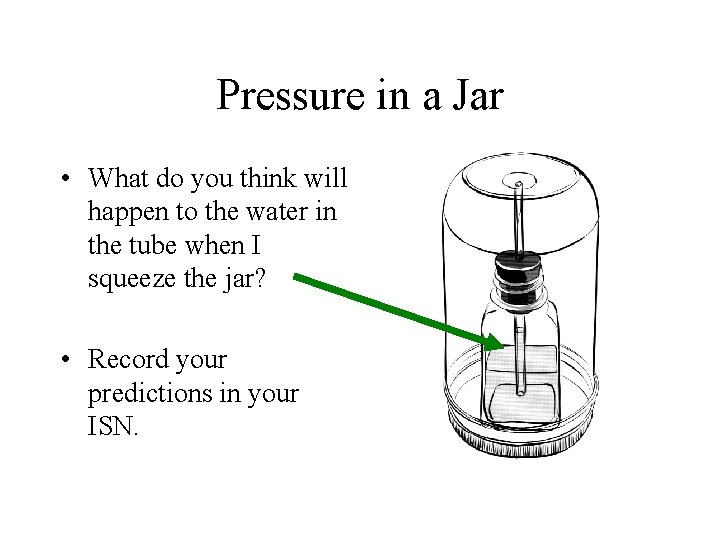
Pressure in a Jar • What do you think will happen to the water in the tube when I squeeze the jar? • Record your predictions in your ISN.

Gently Squeeze the Jar… • What happens to the water level in the tube? • Surprised? • Water level in tube goes down!! • Explain why this happens!

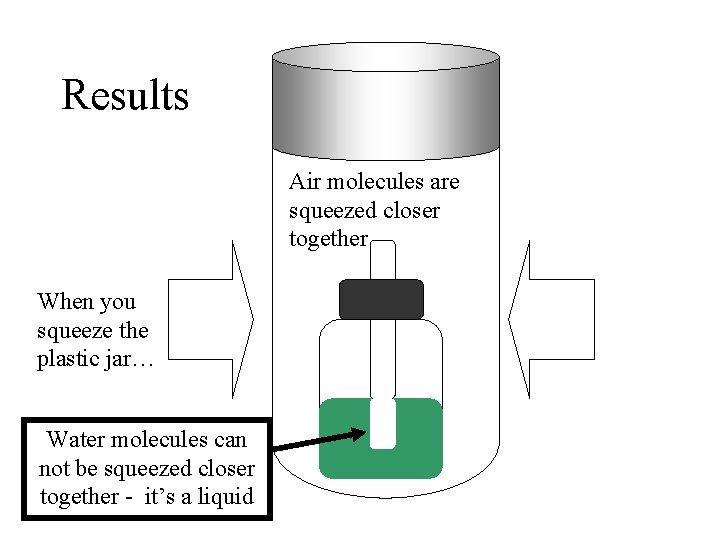
Results Air molecules are squeezed closer together When you squeeze the plastic jar… Water molecules can not be squeezed closer together - it’s a liquid

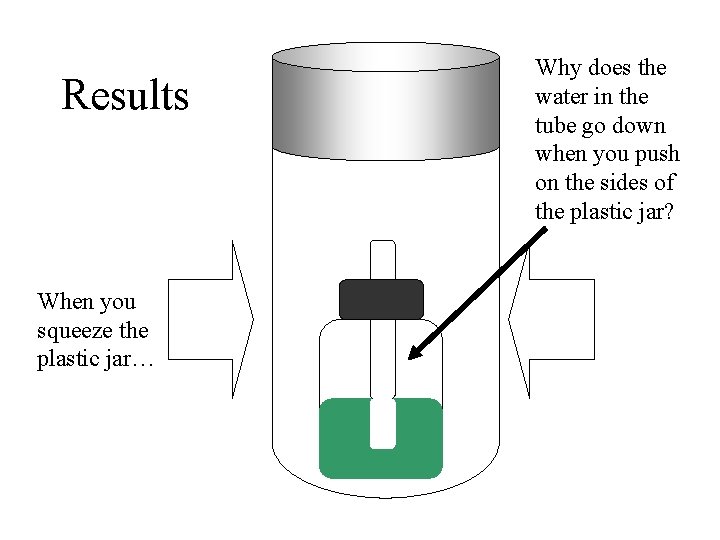
Results When you squeeze the plastic jar… Why does the water in the tube go down when you push on the sides of the plastic jar?

Why it happens… Molecules have less room to move around so they bang into each other and the sides of the jar more often When you squeeze the plastic jar… The volume in the plastic jar is less Increases the pressure in the plastic jar

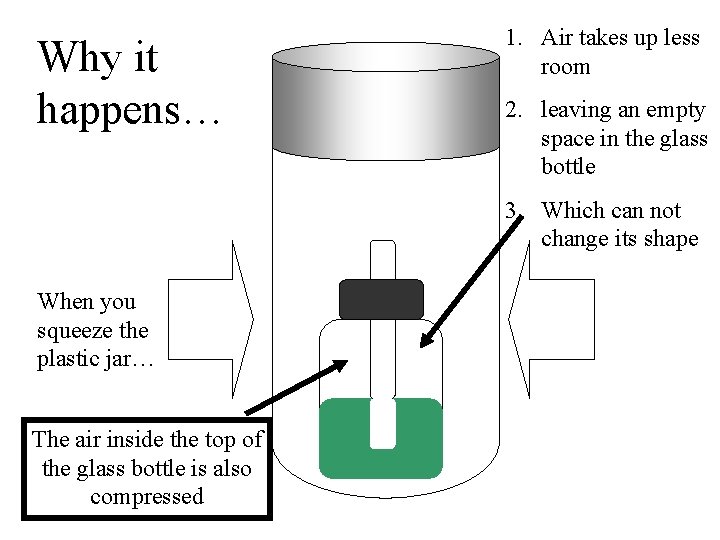
Why it happens… 1. Air takes up less room 2. leaving an empty space in the glass bottle 3. Which can not change its shape When you squeeze the plastic jar… The air inside the top of the glass bottle is also compressed

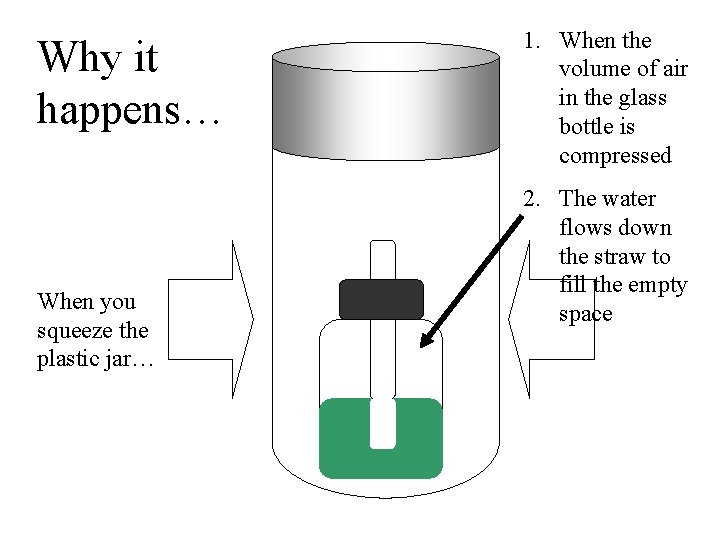
Why it happens… When you squeeze the plastic jar… 1. When the volume of air in the glass bottle is compressed 2. The water flows down the straw to fill the empty space

Question 2 - Page 45(LB) • Decreased the volume in plastic jar and increased air pressure • Air in the glass bottle is compressed leaving an empty space in the glass bottle • Water flowed from the tube to fill the space previously filled with air in the glass bottle

Question 3 - Page 45 (LB) • There would be less pressure • Air in the glass bottle would expand • Pushing the water up the tube

Multimedia • Gas in a syringe simulation • Compare the syringe system to the pressure indicator.

Density • What happened to the volume? • It decreased • What happened to the number of molecules? • Stayed the same.

Density • If the number of molecules stayed the same, what happened to the total mass of the molecules? • It stayed the same. • If the volume decreased and the mass stayed the same, what happened to the density of the air? • The density increased

Reading • Starting on page 48 of your resource book read “What is Air Pressure”

What Is Pressure? • Atmosphere is composed of air • A column of air 1 cm square has a mass of 1. 2 Kg • Atmospheric Pressure: the force applied by the air above you • Molecules have mass so they are pulled to Earth by gravity • Kinetic energy helps us live with pressure • Atmospheric pressure acts with equal force in every direction

Barometer • Measures air pressure • Baro- means pressure • -meter- means measure

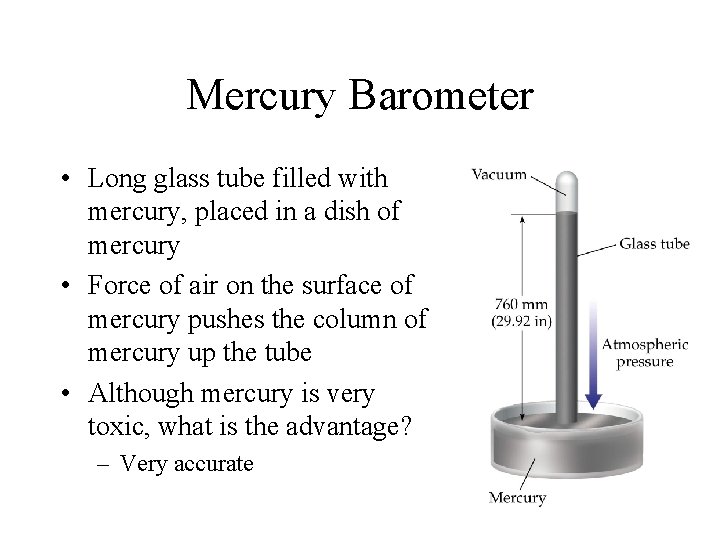
Mercury Barometer • Long glass tube filled with mercury, placed in a dish of mercury • Force of air on the surface of mercury pushes the column of mercury up the tube • Although mercury is very toxic, what is the advantage? – Very accurate

Aneroid Barometer • How does an aneroid barometer work? – As pressure changes, a spring will coil and uncoil and give us a reading in millibars • What is the advantage? – Small and compact

Units • Bars – Used to measure air pressure – Equal to the pressure exerted by air in the atmosphere at sea level • Millibar (mb) – Unit used to measure atmospheric pressure – 1/1000 of a bar – average sea-level air pressure = 1013 mb

Response Sheet-Air Pressure and Wind • Pull out page 47 of your lab book and answer the question.
 Sea breeze usually originates during the
Sea breeze usually originates during the Chapter 19 air pressure and wind
Chapter 19 air pressure and wind Air pressure
Air pressure Winds
Winds What weather instrument measures air temperature
What weather instrument measures air temperature Kurshalter
Kurshalter Air mass vocabulary
Air mass vocabulary Pt tanah air sentosa
Pt tanah air sentosa Coriolis force
Coriolis force Wind pressure gradient
Wind pressure gradient Power force x velocity
Power force x velocity Velocity pressure exposure coefficient
Velocity pressure exposure coefficient West north west wind direction
West north west wind direction Bernoulli's principle pipe flow
Bernoulli's principle pipe flow Osmosis blood pressure
Osmosis blood pressure High pressure and low pressure
High pressure and low pressure Tiefdruckgebiet
Tiefdruckgebiet Area of low pressure where air masses meet and rise
Area of low pressure where air masses meet and rise Relationship between air pressure and density
Relationship between air pressure and density Relationship between air pressure and density
Relationship between air pressure and density Air pressure units
Air pressure units Relationship between air pressure and density
Relationship between air pressure and density Relationship between air pressure and density
Relationship between air pressure and density