V Diffusion in Solids MECE 3345 Materials Science

- Slides: 22

V. Diffusion in Solids MECE 3345 Materials Science VI. Diffusion in Solids copyright © 2008 by Li Sun

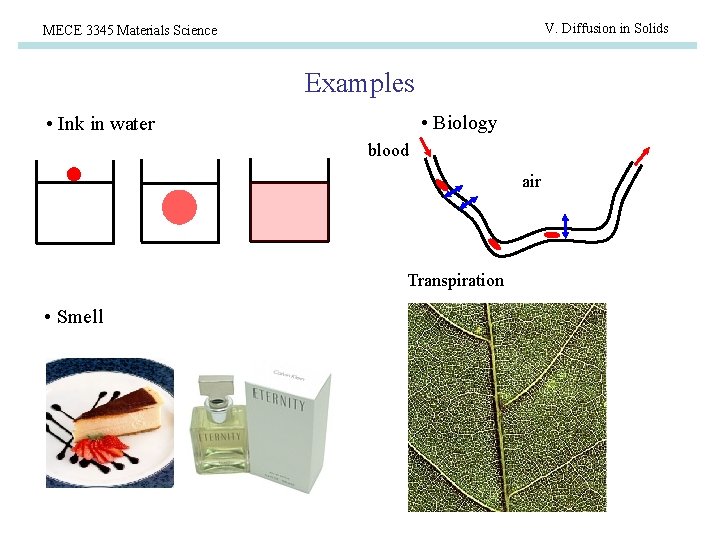
V. Diffusion in Solids MECE 3345 Materials Science Examples • Biology • Ink in water blood air Transpiration • Smell

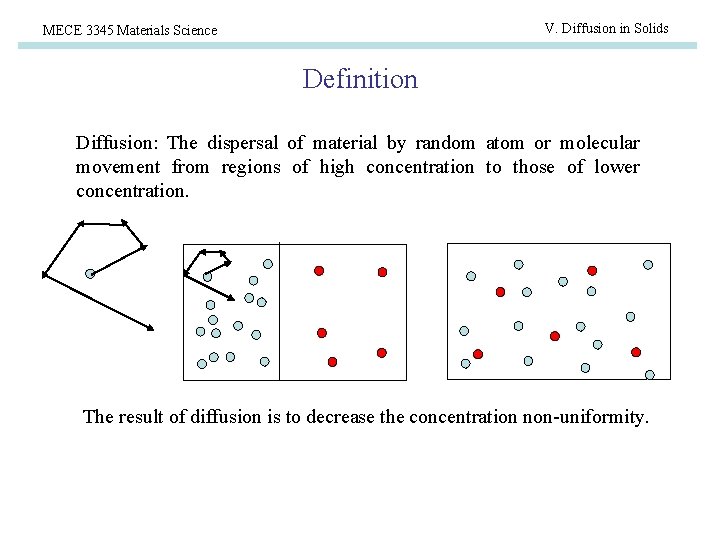
V. Diffusion in Solids MECE 3345 Materials Science Definition Diffusion: The dispersal of material by random atom or molecular movement from regions of high concentration to those of lower concentration. The result of diffusion is to decrease the concentration non-uniformity.

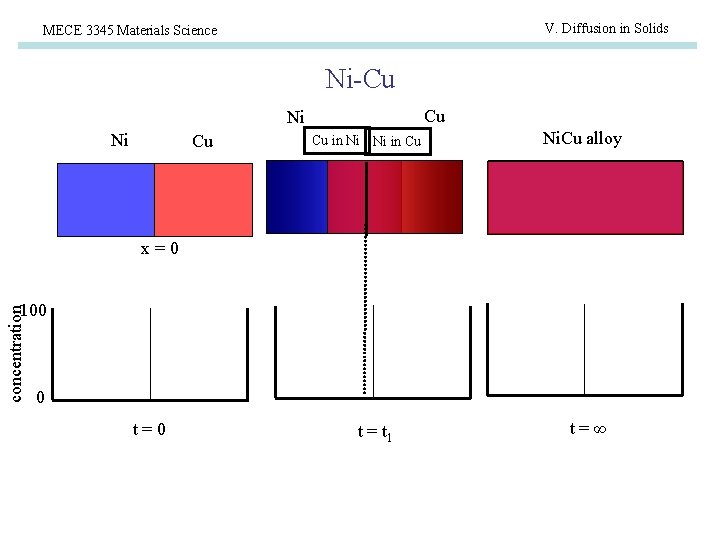
V. Diffusion in Solids MECE 3345 Materials Science Ni-Cu Cu Ni Ni Cu Cu in Ni Ni in Cu Ni. Cu alloy x=0 concentration 100 0 t=0 t = t 1 t=∞

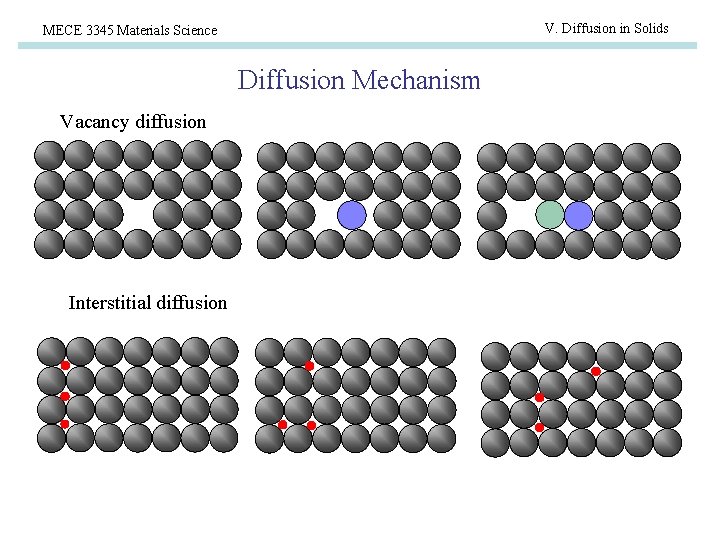
V. Diffusion in Solids MECE 3345 Materials Science Diffusion Mechanism Vacancy diffusion Interstitial diffusion

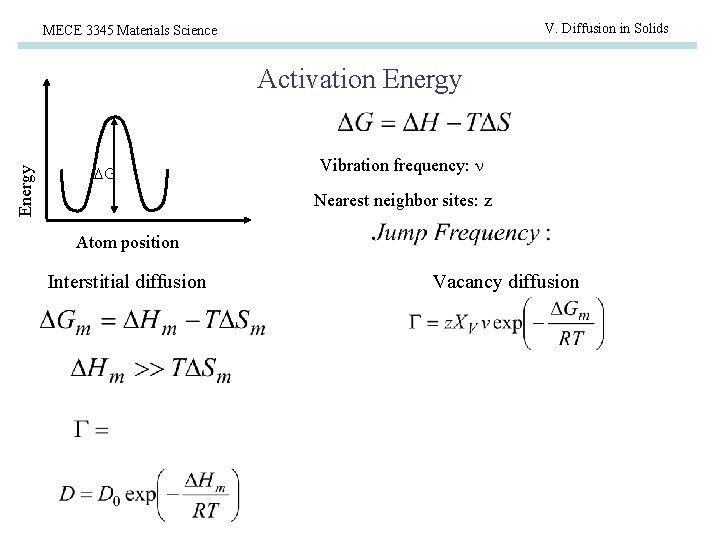
V. Diffusion in Solids MECE 3345 Materials Science Energy Activation Energy G Vibration frequency: Nearest neighbor sites: z Atom position Interstitial diffusion Vacancy diffusion

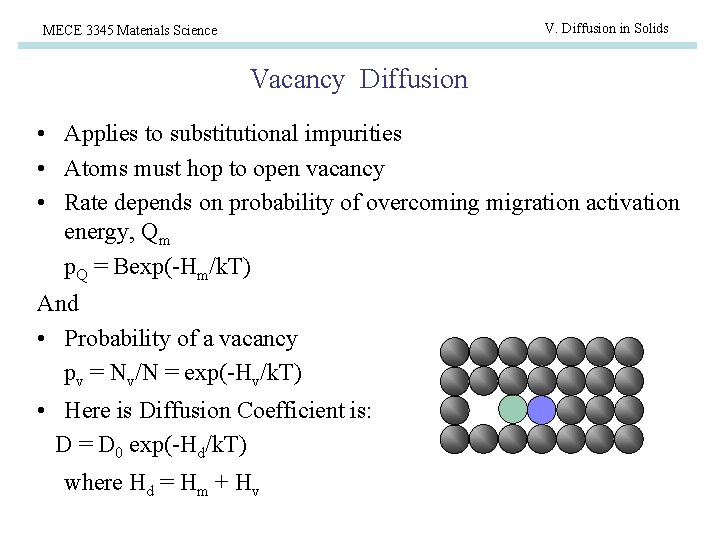
V. Diffusion in Solids MECE 3345 Materials Science Vacancy Diffusion • Applies to substitutional impurities • Atoms must hop to open vacancy • Rate depends on probability of overcoming migration activation energy, Qm p. Q = Bexp(-Hm/k. T) And • Probability of a vacancy pv = Nv/N = exp(-Hv/k. T) • Here is Diffusion Coefficient is: D = D 0 exp(-Hd/k. T) where Hd = Hm + Hv

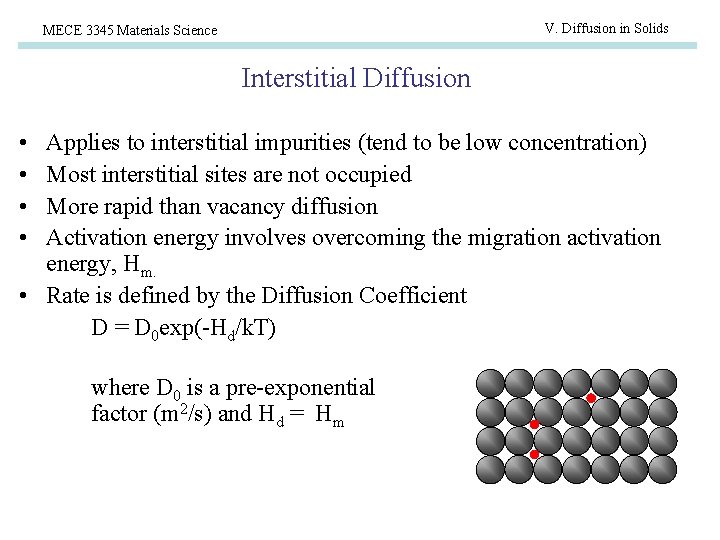
V. Diffusion in Solids MECE 3345 Materials Science Interstitial Diffusion • • Applies to interstitial impurities (tend to be low concentration) Most interstitial sites are not occupied More rapid than vacancy diffusion Activation energy involves overcoming the migration activation energy, Hm. • Rate is defined by the Diffusion Coefficient D = D 0 exp(-Hd/k. T) where D 0 is a pre-exponential factor (m 2/s) and Hd = Hm

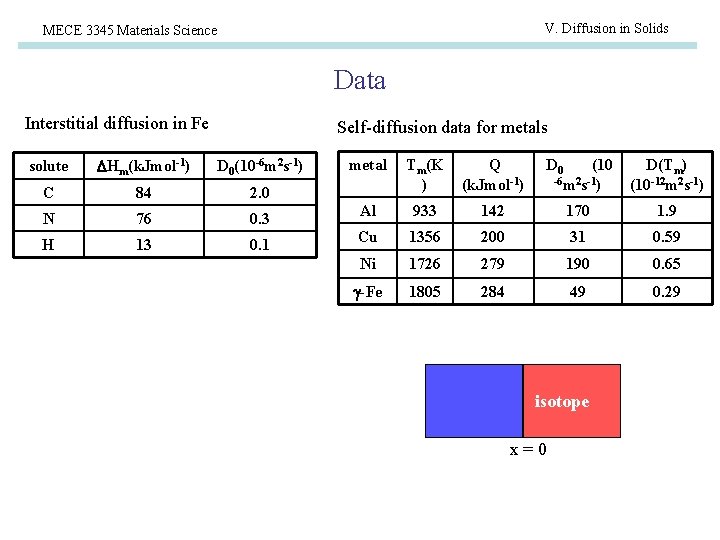
V. Diffusion in Solids MECE 3345 Materials Science Data Interstitial diffusion in Fe Self-diffusion data for metals solute Hm(k. Jmol-1) D 0(10 -6 m 2 s-1) C 84 2. 0 N 76 0. 3 H 13 0. 1 metal Tm(K ) Q (k. Jmol-1) D 0 (10 -6 m 2 s-1) D(Tm) (10 -12 m 2 s-1) Al 933 142 170 1. 9 Cu 1356 200 31 0. 59 Ni 1726 279 190 0. 65 -Fe 1805 284 49 0. 29 isotope x=0

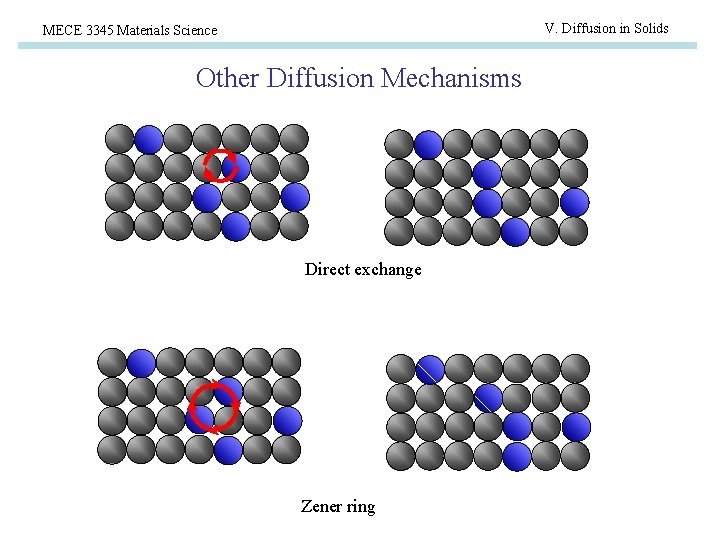
V. Diffusion in Solids MECE 3345 Materials Science Other Diffusion Mechanisms Direct exchange Zener ring

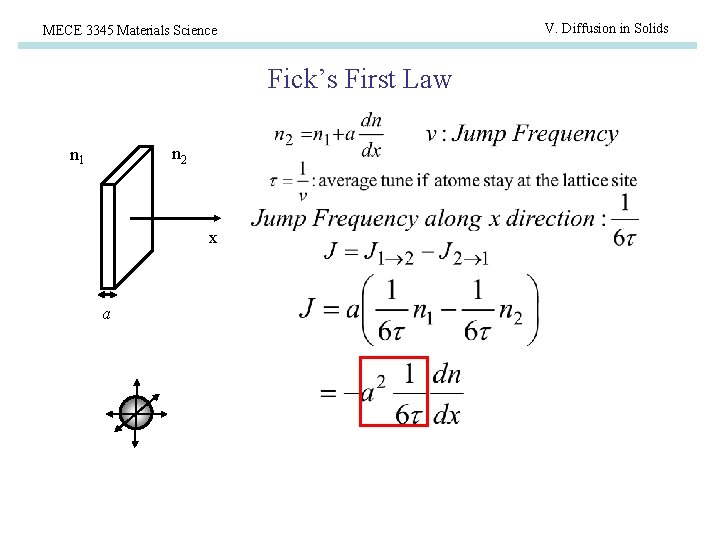
V. Diffusion in Solids MECE 3345 Materials Science Fick’s First Law n 2 n 1 x a

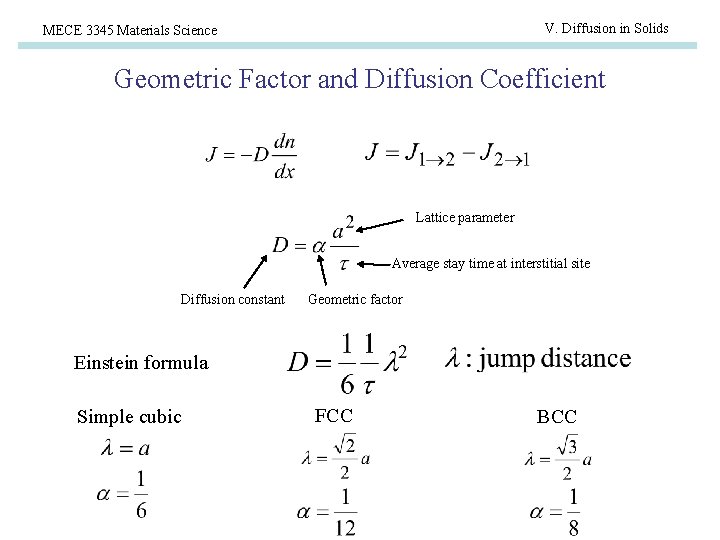
V. Diffusion in Solids MECE 3345 Materials Science Geometric Factor and Diffusion Coefficient Lattice parameter Average stay time at interstitial site Diffusion constant Geometric factor Einstein formula Simple cubic FCC BCC

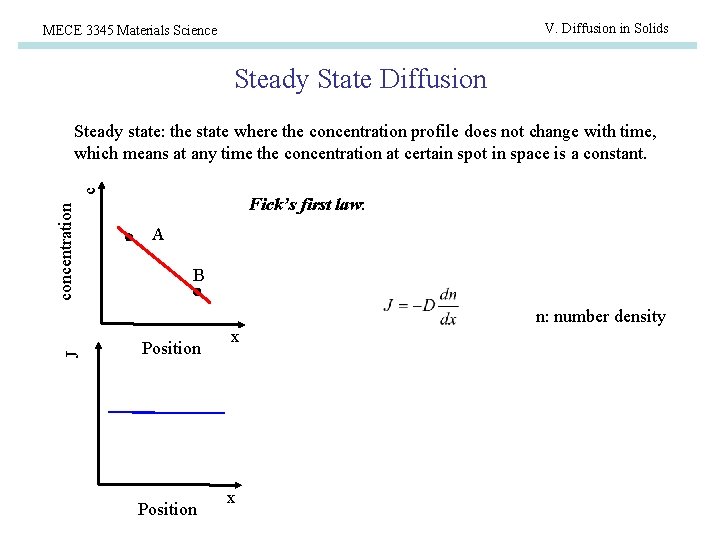
V. Diffusion in Solids MECE 3345 Materials Science Steady State Diffusion concentration c Steady state: the state where the concentration profile does not change with time, which means at any time the concentration at certain spot in space is a constant. Fick’s first law: A B J n: number density Position x x

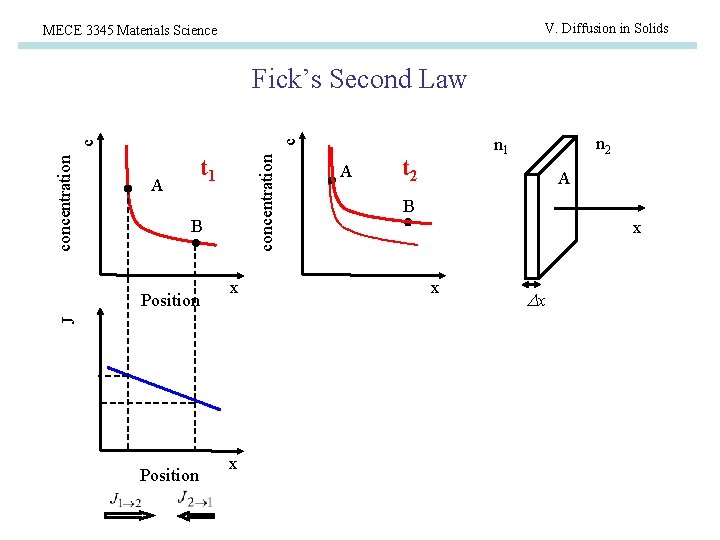
V. Diffusion in Solids MECE 3345 Materials Science Fick’s Second Law c c A concentration t 1 B x J Position x A n 2 n 1 t 2 A B x x x

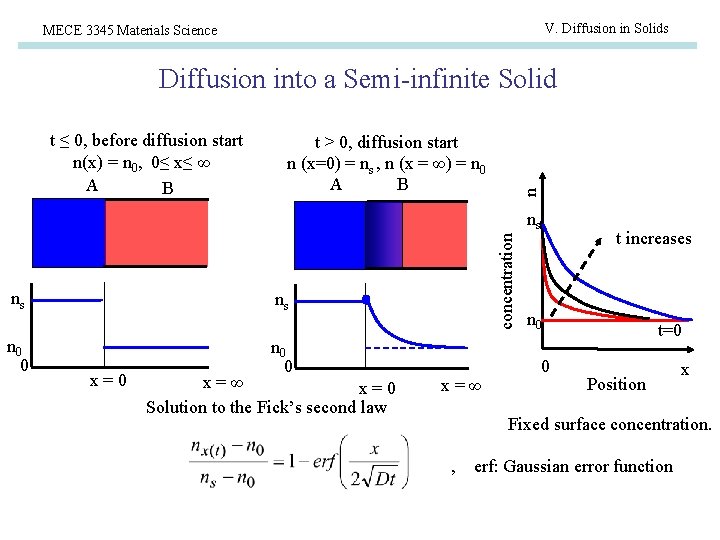
V. Diffusion in Solids MECE 3345 Materials Science Diffusion into a Semi-infinite Solid t > 0, diffusion start n (x=0) = ns , n (x = ∞) = n 0 A B n t ≤ 0, before diffusion start n(x) = n 0, 0≤ x≤ ∞ A B ns n 0 0 concentration ns ns x=0 n 0 0 x=∞ x=0 Solution to the Fick’s second law x=∞ t increases n 0 0 t=0 Position x Fixed surface concentration. , erf: Gaussian error function

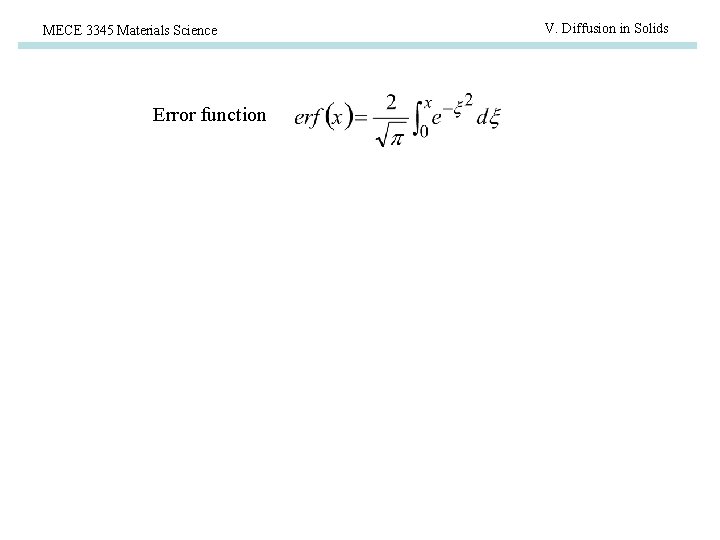
MECE 3345 Materials Science Error function V. Diffusion in Solids

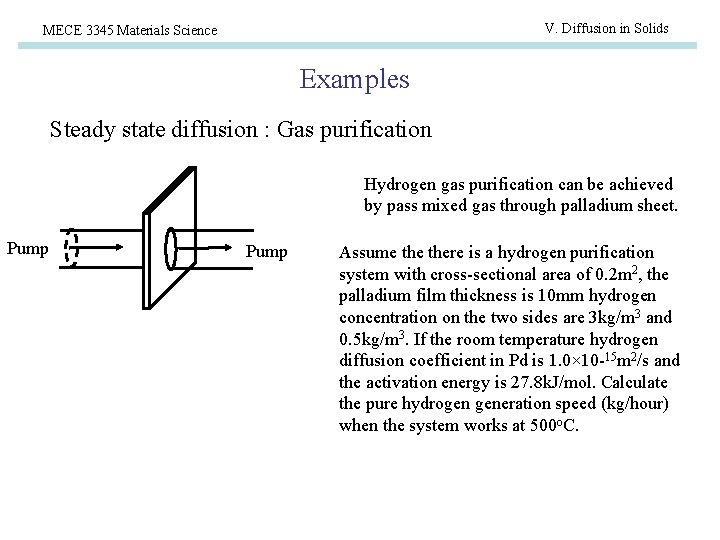
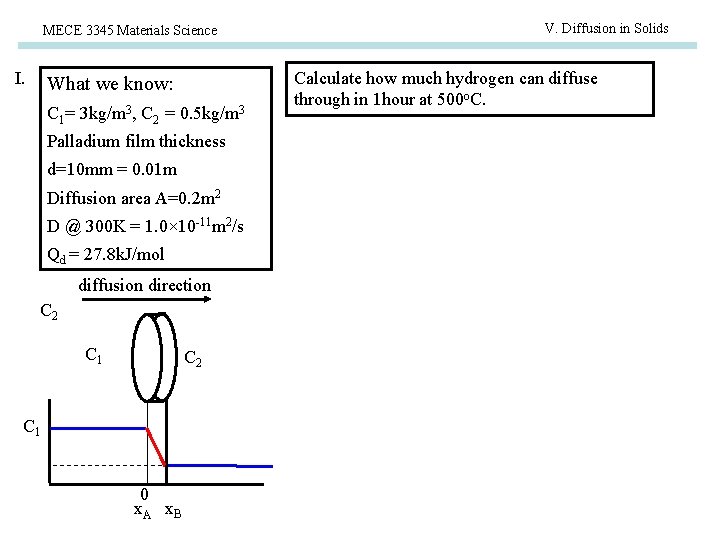
V. Diffusion in Solids MECE 3345 Materials Science Examples Steady state diffusion : Gas purification Hydrogen gas purification can be achieved by pass mixed gas through palladium sheet. Pump Assume there is a hydrogen purification system with cross-sectional area of 0. 2 m 2, the palladium film thickness is 10 mm hydrogen concentration on the two sides are 3 kg/m 3 and 0. 5 kg/m 3. If the room temperature hydrogen diffusion coefficient in Pd is 1. 0× 10 -15 m 2/s and the activation energy is 27. 8 k. J/mol. Calculate the pure hydrogen generation speed (kg/hour) when the system works at 500 o. C.

MECE 3345 Materials Science I. What we know: C 1= 3 kg/m 3, C 2 = 0. 5 kg/m 3 Palladium film thickness d=10 mm = 0. 01 m Diffusion area A=0. 2 m 2 D @ 300 K = 1. 0× 10 -11 m 2/s Qd = 27. 8 k. J/mol diffusion direction C 2 C 1 0 x. A x. B V. Diffusion in Solids Calculate how much hydrogen can diffuse through in 1 hour at 500 o. C.

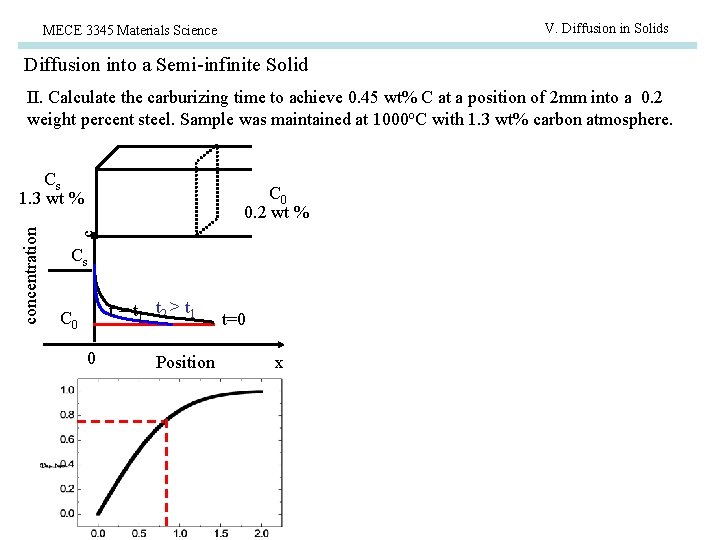
V. Diffusion in Solids MECE 3345 Materials Science Diffusion into a Semi-infinite Solid II. Calculate the carburizing time to achieve 0. 45 wt% C at a position of 2 mm into a 0. 2 weight percent steel. Sample was maintained at 1000 o. C with 1. 3 wt% carbon atmosphere. C 0 0. 2 wt % c concentration Cs 1. 3 wt % Cs t = t 1 t 2 > t 1 C 0 0 Position t=0 x

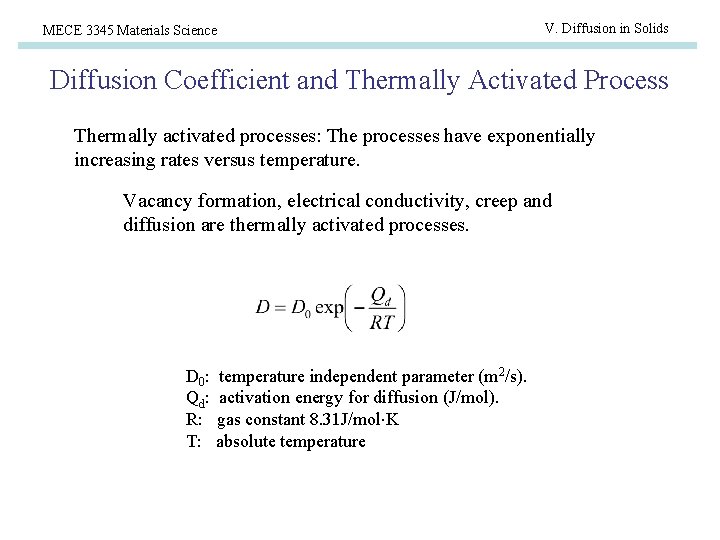
MECE 3345 Materials Science V. Diffusion in Solids Diffusion Coefficient and Thermally Activated Process Thermally activated processes: The processes have exponentially increasing rates versus temperature. Vacancy formation, electrical conductivity, creep and diffusion are thermally activated processes. D 0 : Qd : R: T: temperature independent parameter (m 2/s). activation energy for diffusion (J/mol). gas constant 8. 31 J/mol K absolute temperature

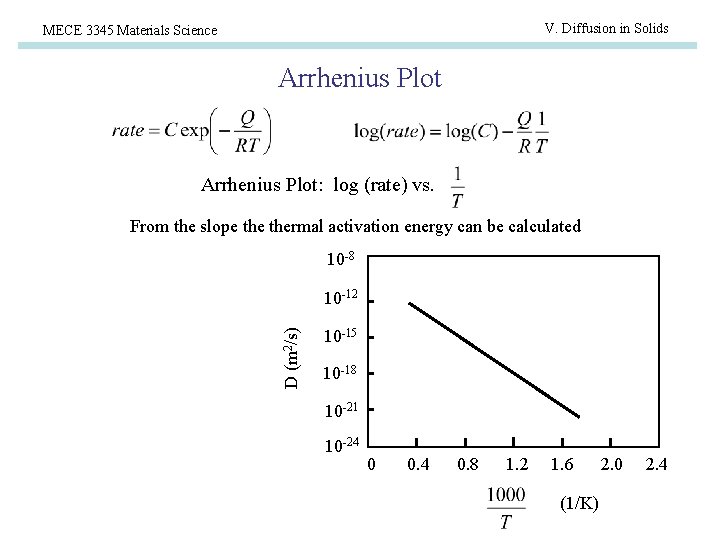
V. Diffusion in Solids MECE 3345 Materials Science Arrhenius Plot: log (rate) vs. From the slope thermal activation energy can be calculated 10 -8 D (m 2/s) 10 -12 10 -15 10 -18 10 -21 10 -24 0 0. 4 0. 8 1. 2 1. 6 (1/K) 2. 0 2. 4

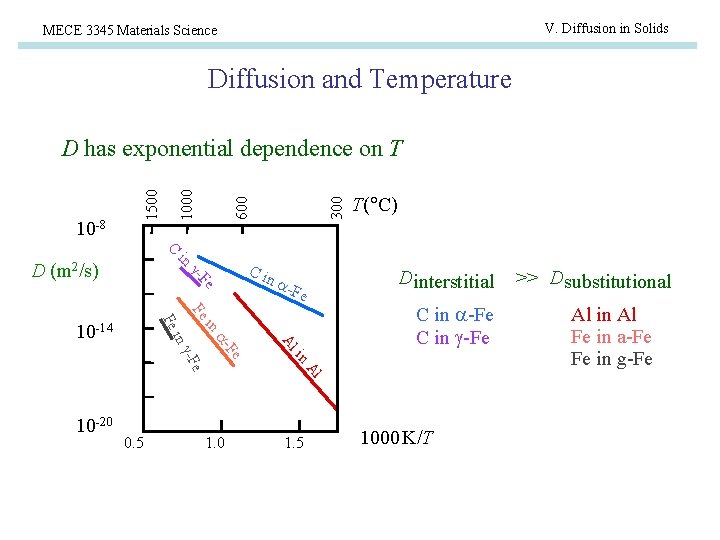
V. Diffusion in Solids MECE 3345 Materials Science Diffusion and Temperature 300 600 10 -8 1500 D has exponential dependence on T T( C) C in D (m 2/s) Ci Fe - n Fe in Fe 1. 0 C in -Fe Al e 0. 5 Dinterstitial in Fe -F 10 -20 Al - in 10 -14 -Fe 1. 5 1000 K/T >> D substitutional Al in Al Fe in a-Fe Fe in g-Fe