Osmosis And Diffusion Diffusion Diffusion is the net






- Slides: 18

Osmosis And Diffusion

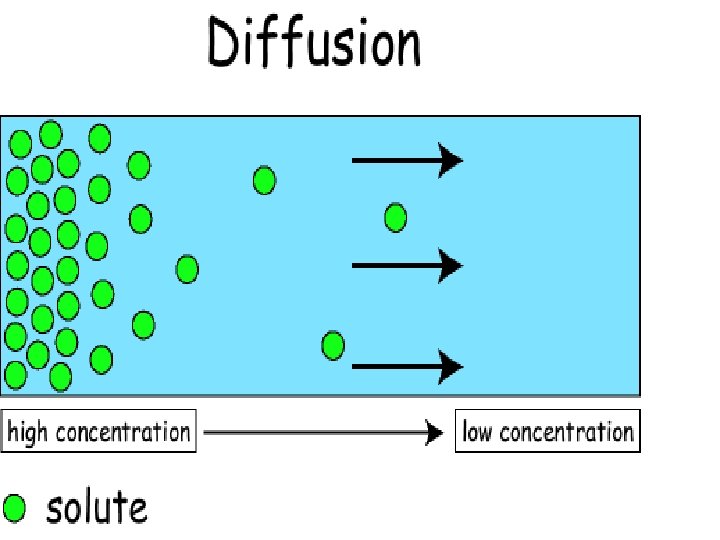
Diffusion Ø Diffusion is - the net movement of molecules from an area of high concentration to an area of lower concentration due to random motion of molecules. Ø ****NO ENERGY NEEDED****


Diffusion Ø This movement of molecules continues until the concentration in all areas are the same and then the motion in and out of that area is equal and the concentration stays the same. Ø This is called Equilibrium

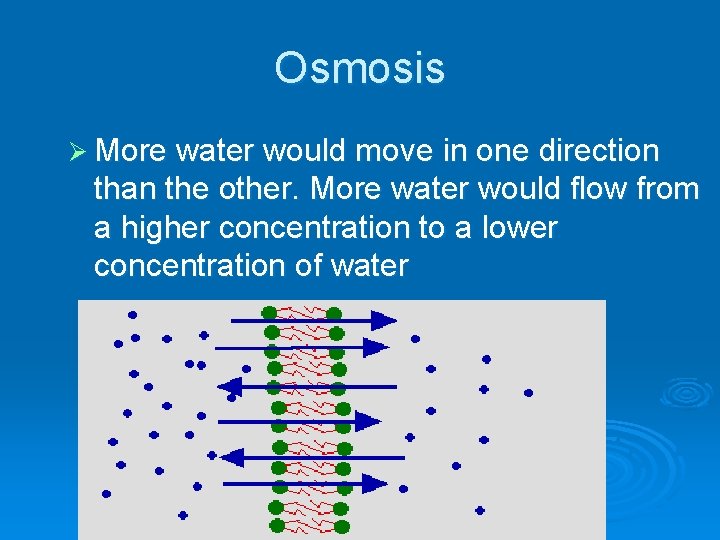
OSMOSIS - no you don’t have to write this stuff yet…. . Ø If there was a membrane with twice as many water molecules on one side as there were on the other (and remember, water can move through the membrane), what do you think would happen

Osmosis Ø More water would move in one direction than the other. More water would flow from a higher concentration to a lower concentration of water

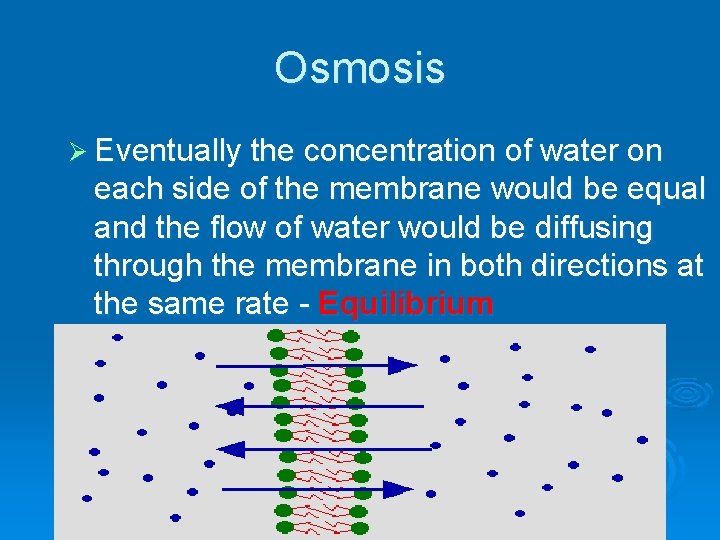
Osmosis Ø Eventually the concentration of water on each side of the membrane would be equal and the flow of water would be diffusing through the membrane in both directions at the same rate - Equilibrium

Osmosis Ø Osmosis- movement of water from area of high concentration to low concentration. Ø Equilibrium- when there is the same concentration of molecules on both sides of a membrane.

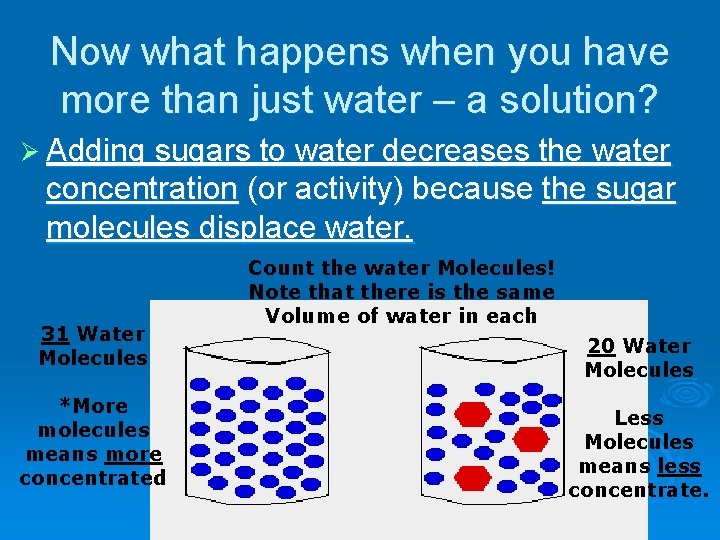
Now what happens when you have more than just water – a solution? Ø Adding sugars to water decreases the water concentration (or activity) because the sugar molecules displace water. 31 Water Molecules *More molecules means more concentrated Count the water Molecules! Note that there is the same Volume of water in each 20 Water Molecules Less Molecules means less concentrate.

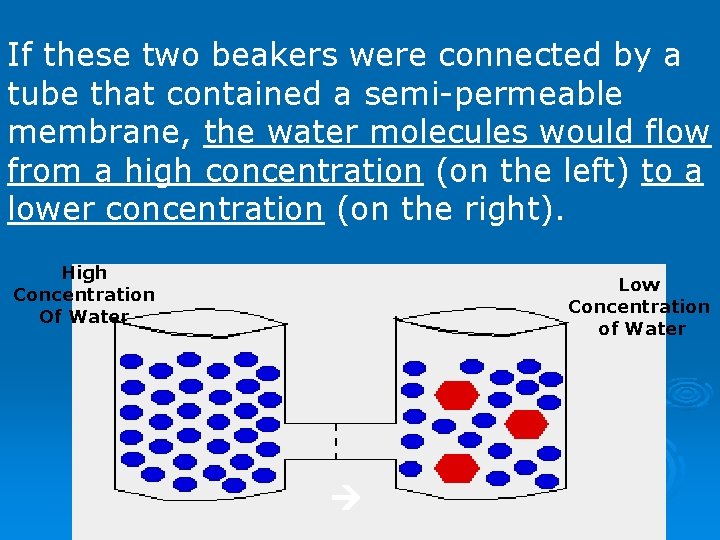
If these two beakers were connected by a tube that contained a semi-permeable membrane, the water molecules would flow from a high concentration (on the left) to a lower concentration (on the right). High Concentration Of Water Low Concentration of Water

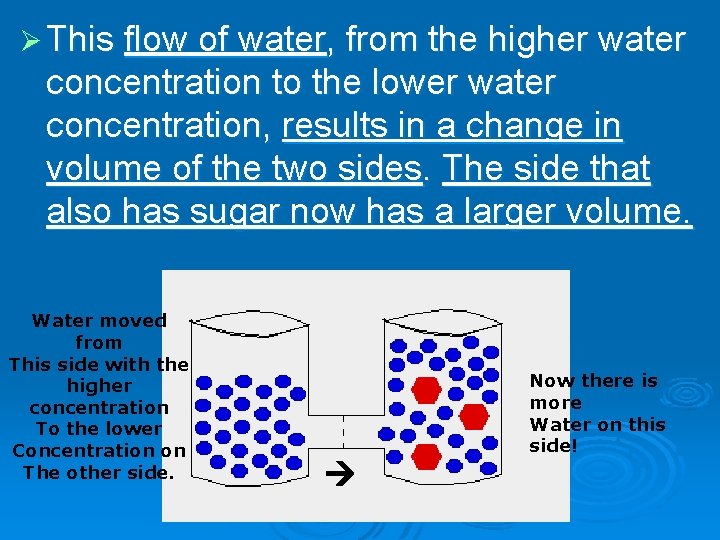
Ø This flow of water, from the higher water concentration to the lower water concentration, results in a change in volume of the two sides. The side that also has sugar now has a larger volume. Water moved from This side with the higher concentration To the lower Concentration on The other side. Now there is more Water on this side!

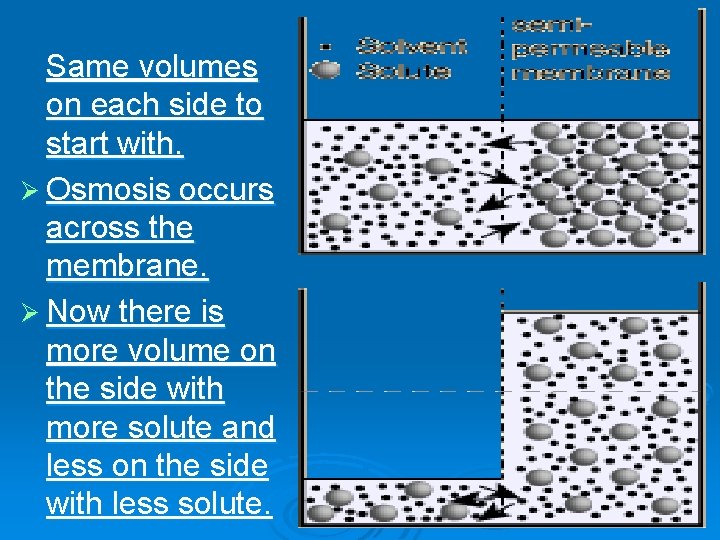
Same volumes on each side to start with. Ø Osmosis occurs across the membrane. Ø Now there is more volume on the side with more solute and less on the side with less solute.

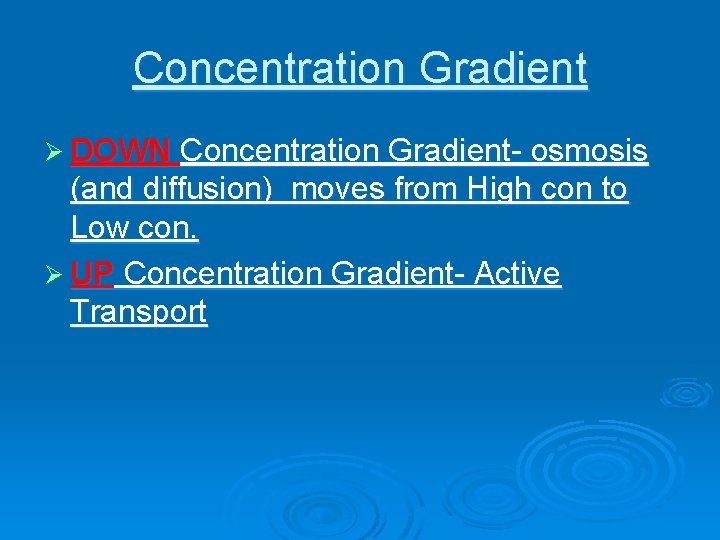
Concentration Gradient Ø DOWN Concentration Gradient- osmosis (and diffusion) moves from High con to Low con. Ø UP Concentration Gradient- Active Transport

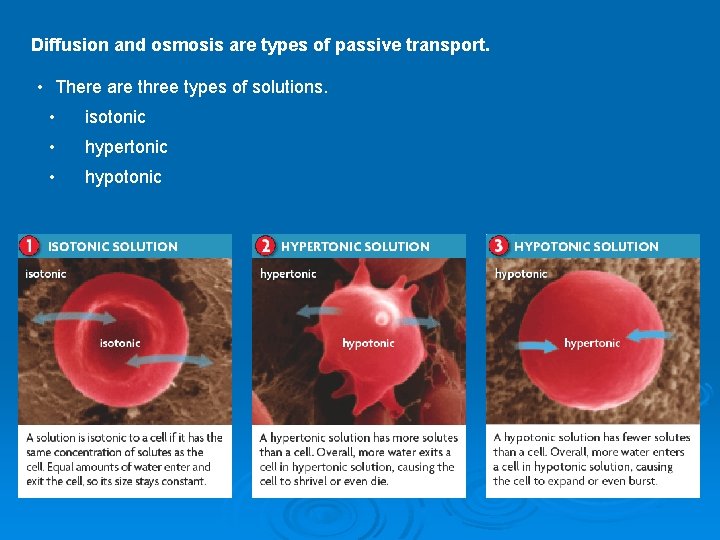
Diffusion and osmosis are types of passive transport. • There are three types of solutions. • isotonic • hypertonic • hypotonic

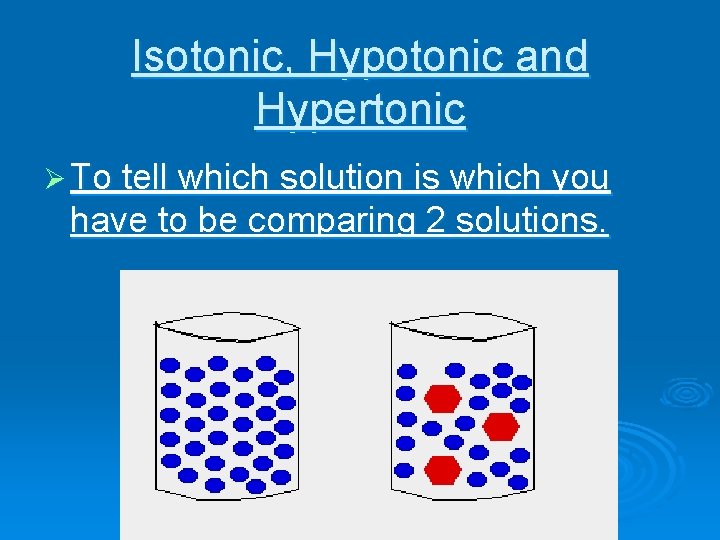
Isotonic, Hypotonic and Hypertonic Ø To tell which solution is which you have to be comparing 2 solutions.

Isotonic Solutions "ISO" means the same Ø If the concentration of solute (salt) is equal on both sides, the water will move back in forth but it won't have any result on the overall amount of water on either side.

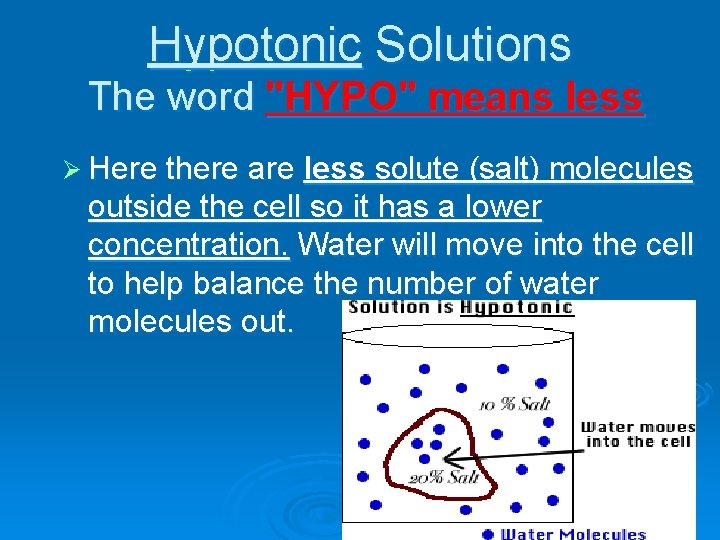
Hypotonic Solutions The word "HYPO" means less Ø Here there are less solute (salt) molecules outside the cell so it has a lower concentration. Water will move into the cell to help balance the number of water molecules out.

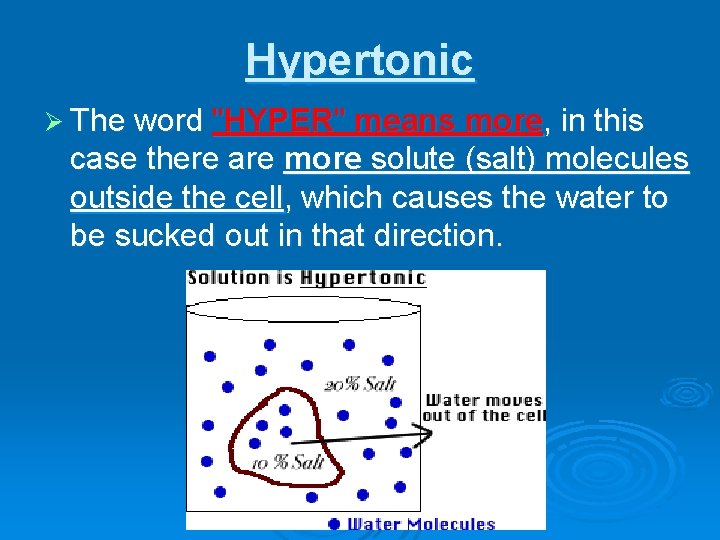
Hypertonic Ø The word "HYPER" means more, in this case there are more solute (salt) molecules outside the cell, which causes the water to be sucked out in that direction.