CHE 333 CLASS 20 DIFFUSION DIFFUSION Diffusion is


- Slides: 12

CHE 333 CLASS 20 DIFFUSION

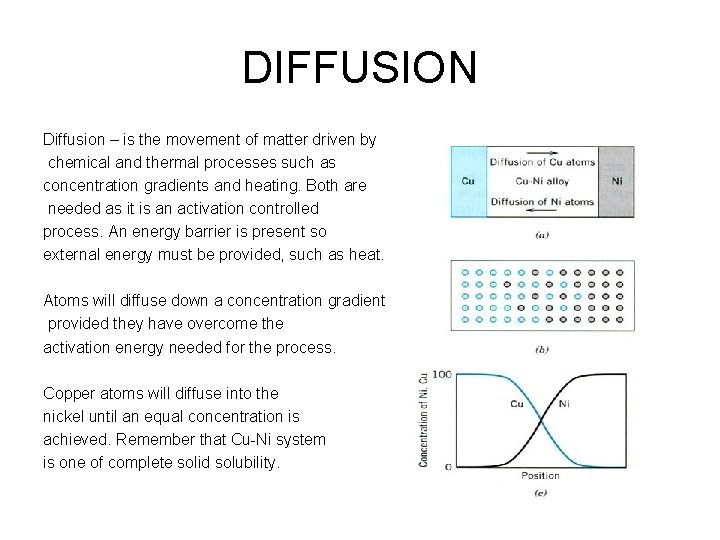
DIFFUSION Diffusion – is the movement of matter driven by chemical and thermal processes such as concentration gradients and heating. Both are needed as it is an activation controlled process. An energy barrier is present so external energy must be provided, such as heat. Atoms will diffuse down a concentration gradient provided they have overcome the activation energy needed for the process. Copper atoms will diffuse into the nickel until an equal concentration is achieved. Remember that Cu-Ni system is one of complete solid solubility.

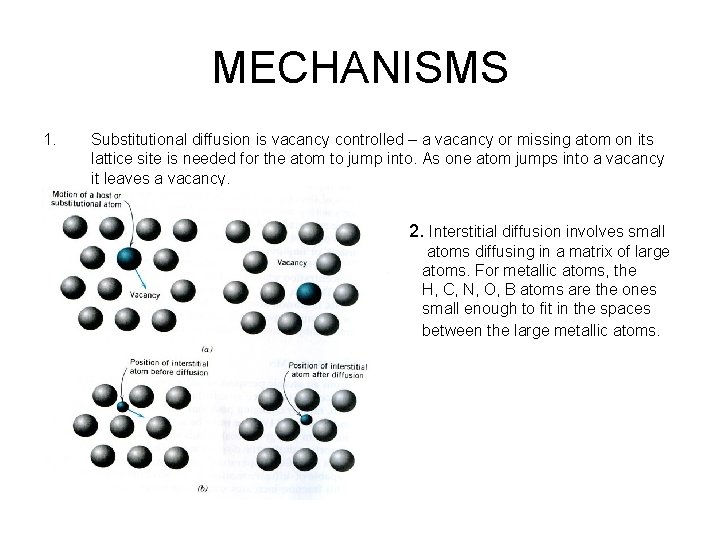
MECHANISMS 1. Substitutional diffusion is vacancy controlled – a vacancy or missing atom on its lattice site is needed for the atom to jump into. As one atom jumps into a vacancy it leaves a vacancy. 2. Interstitial diffusion involves small atoms diffusing in a matrix of large atoms. For metallic atoms, the H, C, N, O, B atoms are the ones small enough to fit in the spaces between the large metallic atoms.

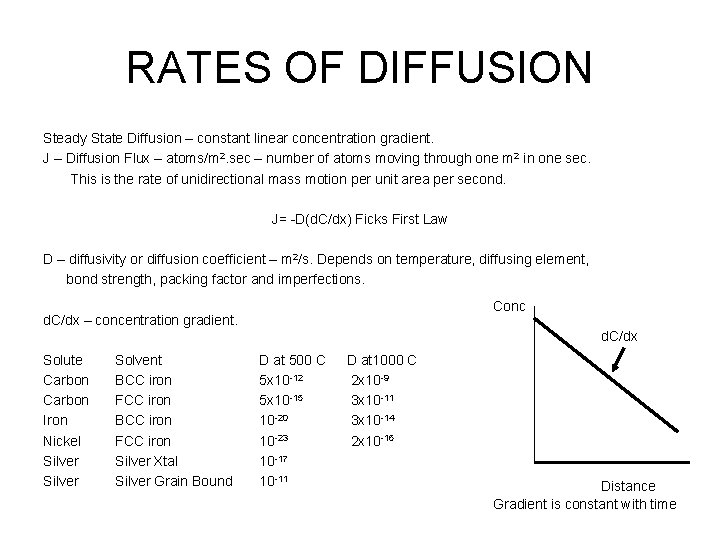
RATES OF DIFFUSION Steady State Diffusion – constant linear concentration gradient. J – Diffusion Flux – atoms/m 2. sec – number of atoms moving through one m 2 in one sec. This is the rate of unidirectional mass motion per unit area per second. J= -D(d. C/dx) Ficks First Law D – diffusivity or diffusion coefficient – m 2/s. Depends on temperature, diffusing element, bond strength, packing factor and imperfections. Conc d. C/dx – concentration gradient. d. C/dx Solute Carbon Iron Nickel Silver Solvent BCC iron FCC iron Silver Xtal Silver Grain Bound D at 500 C 5 x 10 -12 5 x 10 -15 10 -20 10 -23 10 -17 10 -11 D at 1000 C 2 x 10 -9 3 x 10 -11 3 x 10 -14 2 x 10 -16 Distance Gradient is constant with time

Substitutional and Interstitial • From the data, interstitial diffusion is much faster then substitutional diffusion, by several orders of magnitude. Solute Carbon Iron • Solvent BCC iron Type Inter Subst D at 500 C 5 x 10 -12 10 -20 D at 1000 C 2 x 10 -9 3 x 10 -14 Grain boundary diffusion is faster then transgranular diffusion due to the higher number of defects at a grain boundary. This is important in second phase growth under equilibrium conditions, as second phases are then usually found on grain boundaries of the initial phase. For example proeutectoid phases in steels.

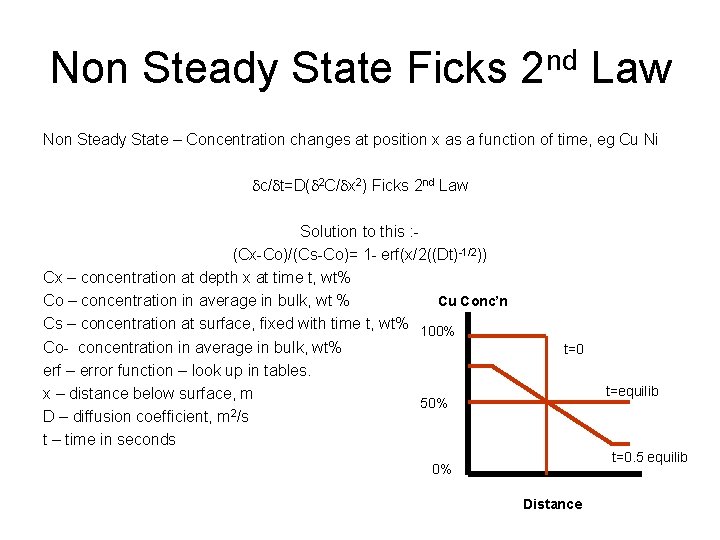
Non Steady State Ficks 2 nd Law Non Steady State – Concentration changes at position x as a function of time, eg Cu Ni dc/dt=D(d 2 C/dx 2) Ficks 2 nd Law Solution to this : (Cx-Co)/(Cs-Co)= 1 - erf(x/2((Dt)-1/2)) Cx – concentration at depth x at time t, wt% Cu Conc’n Co – concentration in average in bulk, wt % Cs – concentration at surface, fixed with time t, wt% 100% Co- concentration in average in bulk, wt% erf – error function – look up in tables. x – distance below surface, m 50% D – diffusion coefficient, m 2/s t – time in seconds t=0 t=equilib t=0. 5 equilib 0% Distance

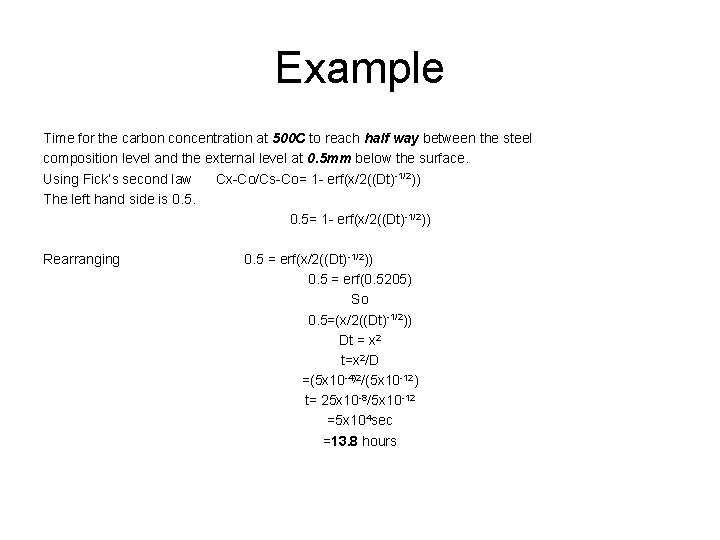
Example Time for the carbon concentration at 500 C to reach half way between the steel composition level and the external level at 0. 5 mm below the surface. Using Fick’s second law Cx-Co/Cs-Co= 1 - erf(x/2((Dt)-1/2)) The left hand side is 0. 5= 1 - erf(x/2((Dt)-1/2)) Rearranging 0. 5 = erf(x/2((Dt) -1/2)) 0. 5 = erf(0. 5205) So 0. 5=(x/2((Dt)-1/2)) Dt = x 2 t=x 2/D =(5 x 10 -4)2/(5 x 10 -12) t= 25 x 10 -8/5 x 10 -12 =5 x 104 sec =13. 8 hours

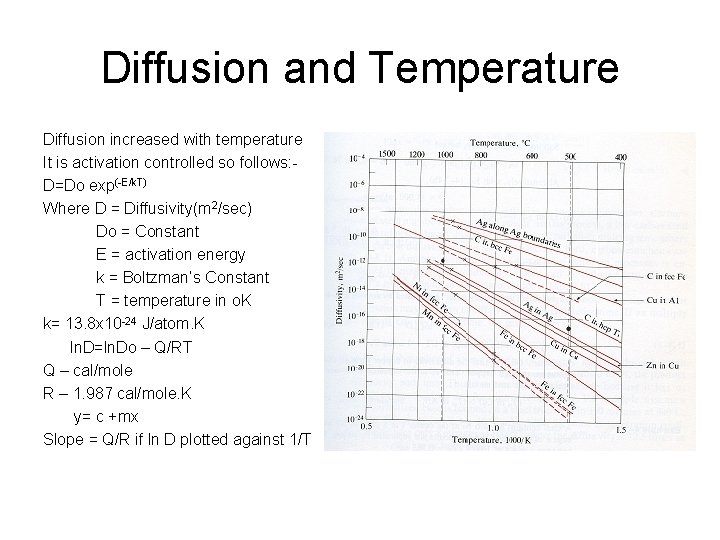
Diffusion and Temperature Diffusion increased with temperature It is activation controlled so follows: D=Do exp(-E/k. T) Where D = Diffusivity(m 2/sec) Do = Constant E = activation energy k = Boltzman’s Constant T = temperature in o. K k= 13. 8 x 10 -24 J/atom. K ln. D=ln. Do – Q/RT Q – cal/mole R – 1. 987 cal/mole. K y= c +mx Slope = Q/R if ln D plotted against 1/T

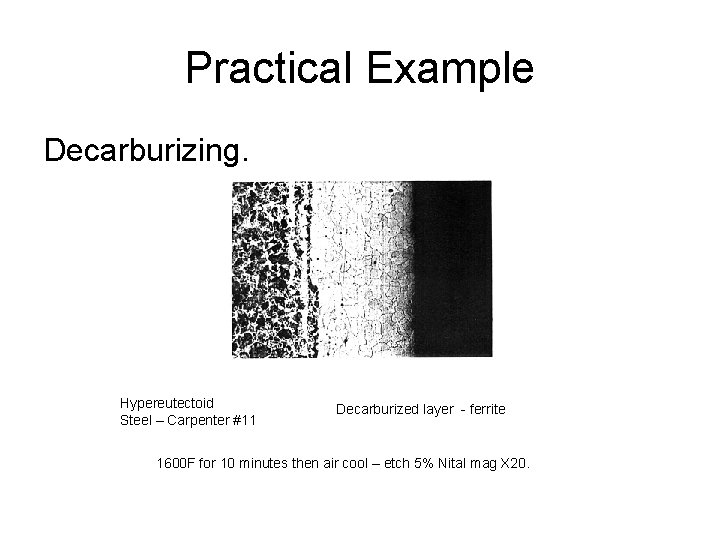
Practical Example Decarburizing. Hypereutectoid Steel – Carpenter #11 Decarburized layer - ferrite 1600 F for 10 minutes then air cool – etch 5% Nital mag X 20.

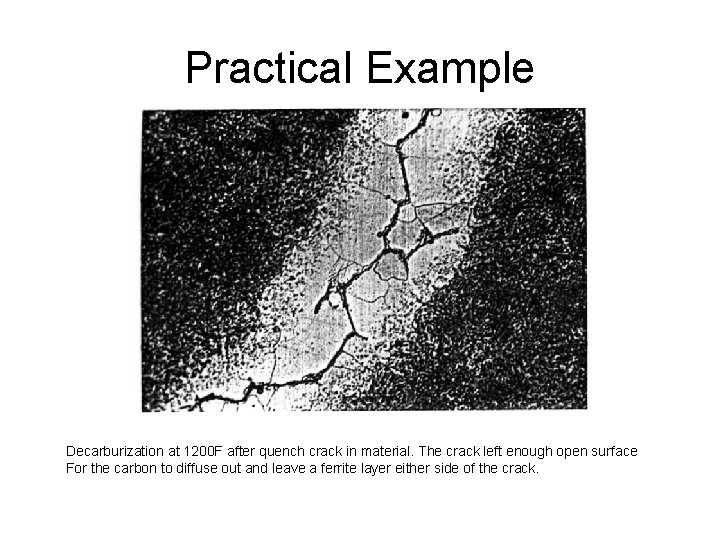
Practical Example Decarburization at 1200 F after quench crack in material. The crack left enough open surface For the carbon to diffuse out and leave a ferrite layer either side of the crack.

Other Applications Bonding – by placing metals close together and heating them, as atoms from one go into the other, a bond is formed. Nitriding, carburizing, for surface hardening of steels – forms hard compounds on the Surface for wear resistance. Removal of hydrogen after electroplating – heat up to 350 F for 24 hours to reduce hydrogen and stop hydrogen embrittlement in high strength steels. Semi conductor processing – dopants added by diffusion to silicon wafers. Vacuum heat treating of titanium – oxygen embrittles titanium so use a vacuum atmosphere or remove a surface layer calculated from Ficks second law. Fuel Cells – Proton Exchange Membrane hydrogen ion diffuses through a polymer. Pharmaceutical drug delivery – controlled release through polymers, creates steady flow compared to tablets which have a high initial amount then quickly low.

Materials Different Materials and Diffusion Rates Metals in metals slow, interstitials in metals much faster. Polymers – Fick’s Laws observed, fast diffusion, for example moisture into polymers 1. 5% weight gain into “free volume” as it is not a crystal structure. Ceramics – very low near zero diffusion rates – ionic and covalent bonding. Composites –orientation dependent, along fiber interfaces high. Damaging – decarburization, oxygen in titanium alloys, hydrogen in steels, oxygen and nitrogen along grain boundaries in metals at high temperature, moisture pick up in composites. Useful Surface treatments of metals, for example carburizing, nitriding Porous materials, lubricant impregnated bearings. Permeation – like diffusion but use volume defects. Concrete – moisture, salt, leads to steel corrosion – Fick’s second law.