University of Torino Department of Oncology Adjuvant Therapy




- Slides: 40

University of Torino – Department of Oncology Adjuvant Therapy for Early Stage Lung Cancer Giorgio V. Scagliotti University of Torino Department of Oncology giorgio. scagliotti@unito. it

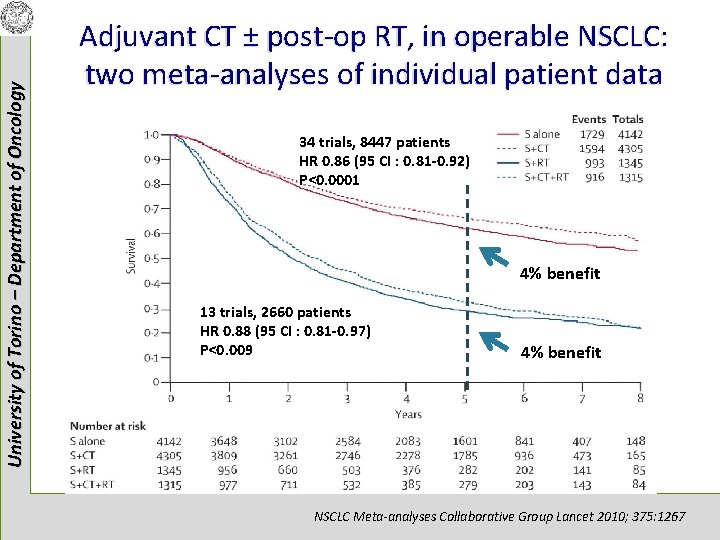
University of Torino – Department of Oncology Adjuvant CT ± post-op RT, in operable NSCLC: two meta-analyses of individual patient data 34 trials, 8447 patients HR 0. 86 (95 CI : 0. 81 -0. 92) P<0. 0001 4% benefit 13 trials, 2660 patients HR 0. 88 (95 CI : 0. 81 -0. 97) P<0. 009 4% benefit NSCLC Meta-analyses Collaborative Group Lancet 2010; 375: 1267

University of Torino – Department of Oncology Adjuvant Chemotherapy in Early Stage NSCLC • Accepted as the standard in node-positive curatively resected early stage NSCLC • Remains controversial in node-negative early stage NSCLC (decisions based on tumor size) • Cisplatin-based therapy considered the standard • Carboplatin often used but “unproven” • Most evidence-based regimen - cis/vinorelbine • ECOG 1505 - allowed other regimens all of which were previously untested in the phase III setting

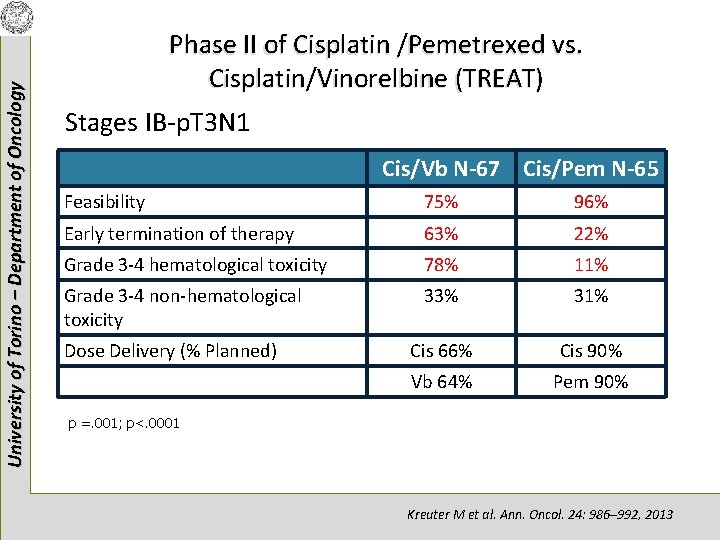
University of Torino – Department of Oncology Phase II of Cisplatin /Pemetrexed vs. Cisplatin/Vinorelbine (TREAT) Stages IB-p. T 3 N 1 Cis/Vb N-67 Cis/Pem N-65 Feasibility 75% 96% Early termination of therapy 63% 22% Grade 3 -4 hematological toxicity 78% 11% Grade 3 -4 non-hematological toxicity 33% 31% Cis 66% Cis 90% Vb 64% Pem 90% Dose Delivery (% Planned) p =. 001; p<. 0001 Kreuter M et al. Ann. Oncol. 24: 986– 992, 2013

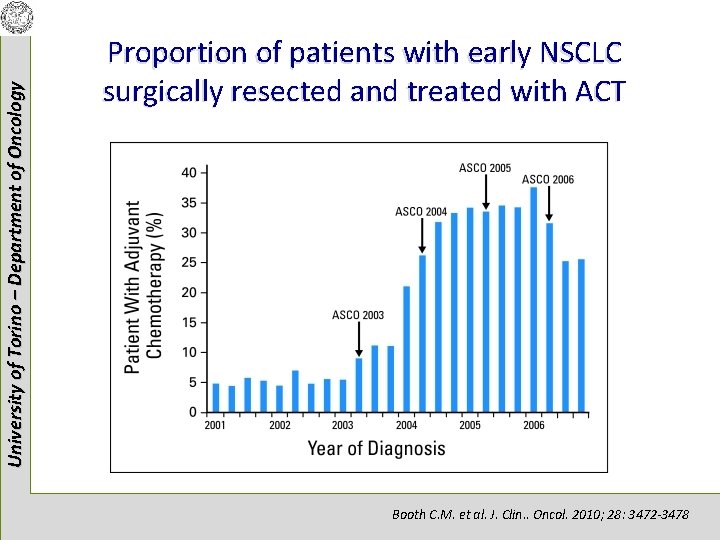
University of Torino – Department of Oncology Proportion of patients with early NSCLC surgically resected and treated with ACT Booth C. M. et al. J. Clin. . Oncol. 2010; 28: 3472 -3478

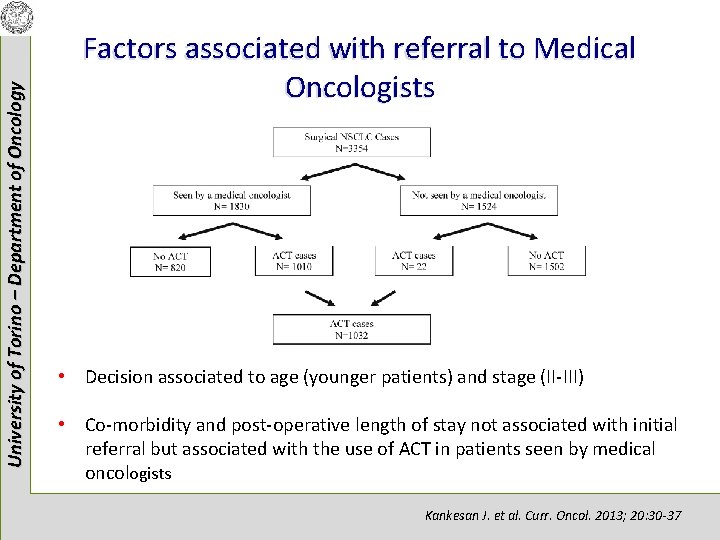
University of Torino – Department of Oncology Factors associated with referral to Medical Oncologists • Decision associated to age (younger patients) and stage (II-III) • Co-morbidity and post-operative length of stay not associated with initial referral but associated with the use of ACT in patients seen by medical oncologists Kankesan J. et al. Curr. Oncol. 2013; 20: 30 -37

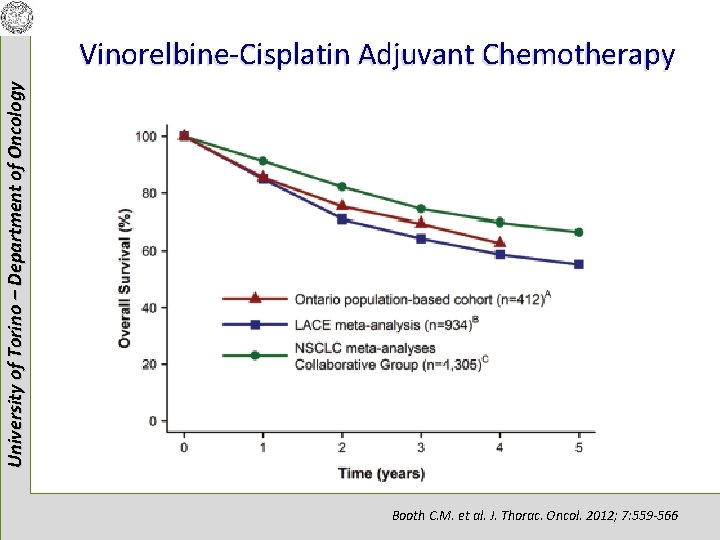
University of Torino – Department of Oncology Vinorelbine-Cisplatin Adjuvant Chemotherapy Booth C. M. et al. J. Thorac. Oncol. 2012; 7: 559 -566

University of Torino – Department of Oncology Compliance and Toxicity of Adjuvant Chemotherapy • In ACT the most evidence-based regimen is cisplatin/vinorelbine • Weekly schedule of vinorelbine used in some of the phase III studies was associated with low compliance and significant toxicities. • Discrepancies with routine clinical practice. • Carboplatin often used but “unproven”. • Need for studies testing alternative regimens and schedules.

University of Torino – Department of Oncology Hurdles in adjuvant studies in NSCLC • Phase II “proof of concept” studies less applicable to adjuvant setting. • In adjuvant studies overall response rate is NOT an endpoint. • Survival is much longer and potentially impacted by additional lines of therapy at relapse. • Quality of life issues and adverse events. • Early stage NSCLC are less frequently reported than in other types of tumors (e. g. breast).

University of Torino – Department of Oncology Adjuvant Trials • Primary endpoint – Disease free survival vs. Overall survival? • Cross over effect – Disease free survival- 2 years? 3 years? – Event free survival

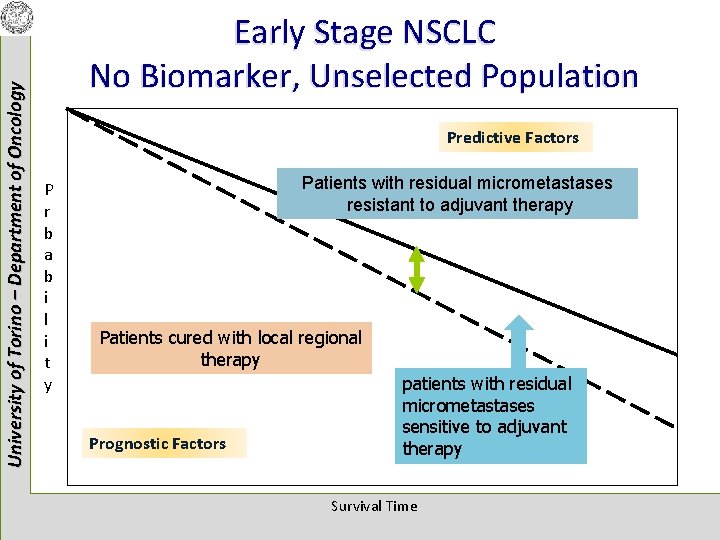
University of Torino – Department of Oncology Early Stage NSCLC No Biomarker, Unselected Population Predictive Factors P r b a b i l i t y Patients with residual micrometastases resistant to adjuvant therapy Patients cured with local regional therapy Prognostic Factors patients with residual micrometastases sensitive to adjuvant therapy Survival Time

University of Torino – Department of Oncology How Can We Improve Cure Rates for Early Stage NSCLC? • Better risk stratification to guide selection of neo-adjuvant & adjuvant therapy – Develop novel options for high-risk patients • Tailoring chemotherapy based on individual molecular markers – ERCC 1, BRCA 1, RRM 1, TS, genomic alterations • Integration of targeted agents

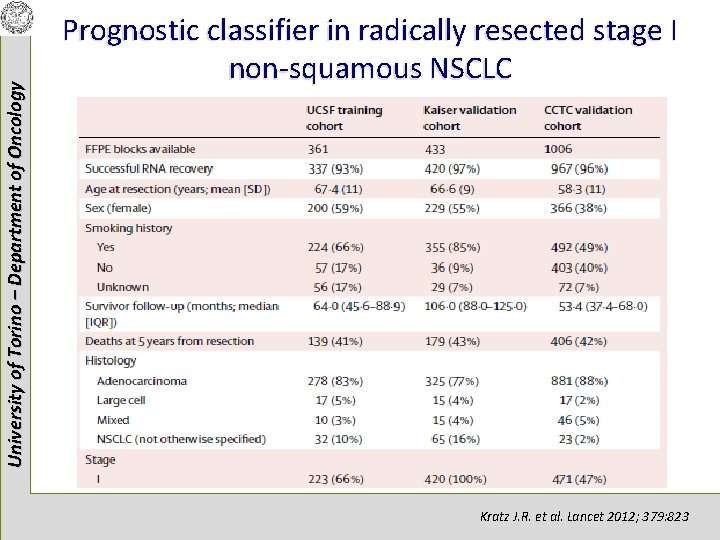
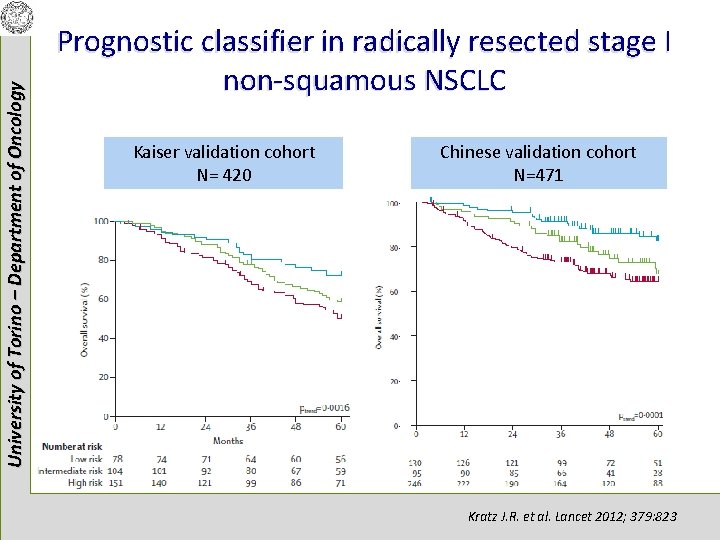
University of Torino – Department of Oncology Prognostic classifier in radically resected stage I non-squamous NSCLC Kratz J. R. et al. Lancet 2012; 379: 823

University of Torino – Department of Oncology Prognostic classifier in radically resected stage I non-squamous NSCLC Kaiser validation cohort N= 420 Chinese validation cohort N=471 Kratz J. R. et al. Lancet 2012; 379: 823

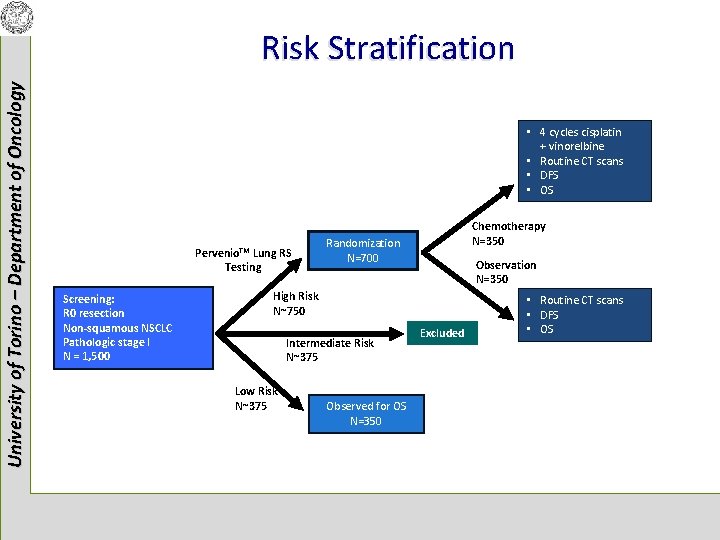
University of Torino – Department of Oncology Risk Stratification • 4 cycles cisplatin + vinorelbine • Routine CT scans • DFS • OS Pervenio. TM Lung RS Testing Screening: R 0 resection Non-squamous NSCLC Pathologic stage I N = 1, 500 Chemotherapy N=350 Randomization N=700 Observation N=350 High Risk N~750 Intermediate Risk N~375 Low Risk N~375 Observed for OS N=350 Excluded • Routine CT scans • DFS • OS

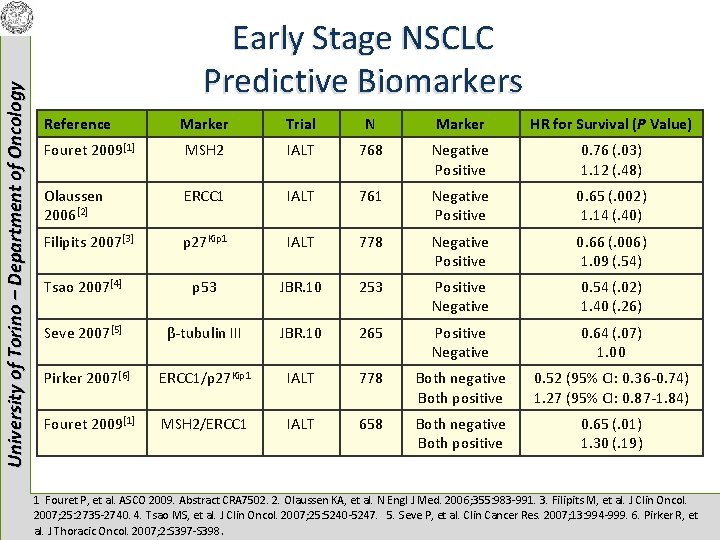
University of Torino – Department of Oncology Early Stage NSCLC Predictive Biomarkers Reference Marker Trial N Marker HR for Survival (P Value) Fouret 2009[1] MSH 2 IALT 768 Negative Positive 0. 76 (. 03) 1. 12 (. 48) Olaussen 2006[2] ERCC 1 IALT 761 Negative Positive 0. 65 (. 002) 1. 14 (. 40) Filipits 2007[3] p 27 Kip 1 IALT 778 Negative Positive 0. 66 (. 006) 1. 09 (. 54) Tsao 2007[4] p 53 JBR. 10 253 Positive Negative 0. 54 (. 02) 1. 40 (. 26) Seve 2007[5] β-tubulin III JBR. 10 265 Positive Negative 0. 64 (. 07) 1. 00 Pirker 2007[6] ERCC 1/p 27 Kip 1 IALT 778 Both negative Both positive 0. 52 (95% CI: 0. 36 -0. 74) 1. 27 (95% CI: 0. 87 -1. 84) Fouret 2009[1] MSH 2/ERCC 1 IALT 658 Both negative Both positive 0. 65 (. 01) 1. 30 (. 19) 1. Fouret P, et al. ASCO 2009. Abstract CRA 7502. 2. Olaussen KA, et al. N Engl J Med. 2006; 355: 983 -991. 3. Filipits M, et al. J Clin Oncol. 2007; 25: 2735 -2740. 4. Tsao MS, et al. J Clin Oncol. 2007; 25: 5240 -5247. 5. Seve P, et al. Clin Cancer Res. 2007; 13: 994 -999. 6. Pirker R, et al. J Thoracic Oncol. 2007; 2: S 397 -S 398.

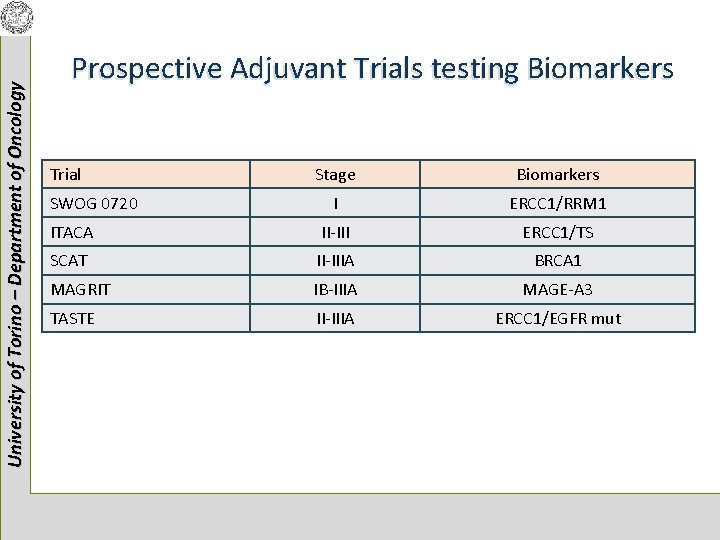
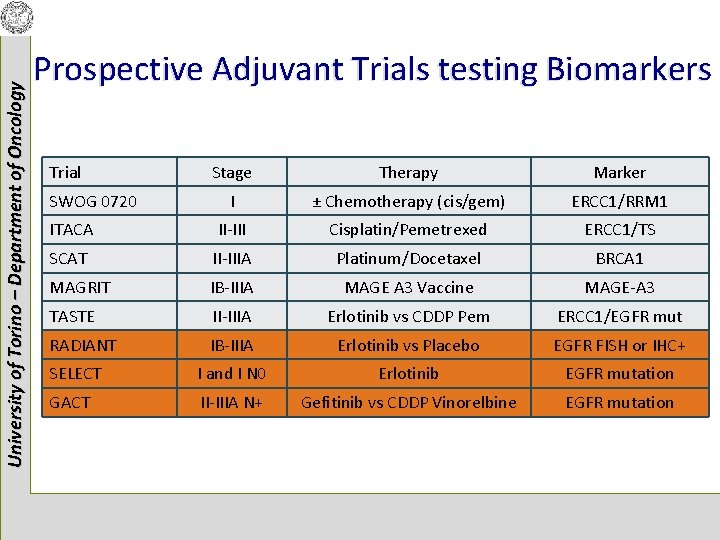
University of Torino – Department of Oncology Prospective Adjuvant Trials testing Biomarkers Trial Stage Biomarkers I ERCC 1/RRM 1 ITACA II-III ERCC 1/TS SCAT II-IIIA BRCA 1 MAGRIT IB-IIIA MAGE-A 3 TASTE II-IIIA ERCC 1/EGFR mut SWOG 0720

University of Torino – Department of Oncology Prospective Adjuvant Trials testing Biomarkers Trial Stage Therapy Marker I ± Chemotherapy (cis/gem) ERCC 1/RRM 1 ITACA II-III Cisplatin/Pemetrexed ERCC 1/TS SCAT II-IIIA Platinum/Docetaxel BRCA 1 MAGRIT IB-IIIA MAGE A 3 Vaccine MAGE-A 3 TASTE II-IIIA Erlotinib vs CDDP Pem ERCC 1/EGFR mut RADIANT IB-IIIA Erlotinib vs Placebo EGFR FISH or IHC+ SELECT I and I N 0 Erlotinib EGFR mutation GACT II-IIIA N+ Gefitinib vs CDDP Vinorelbine EGFR mutation SWOG 0720

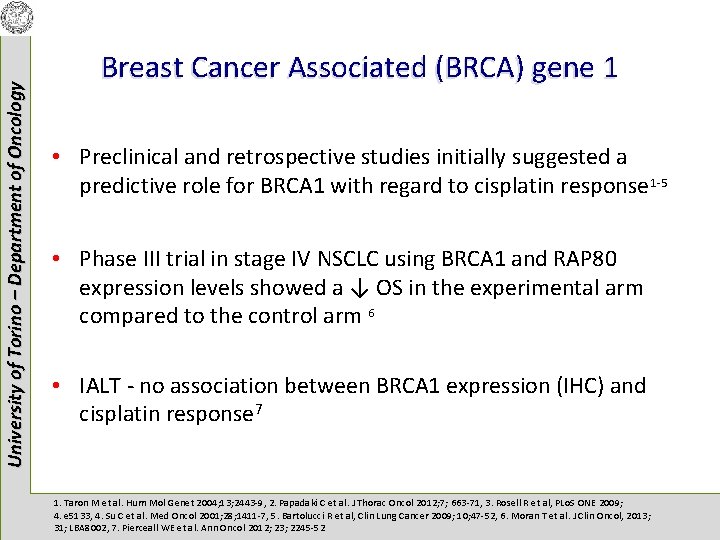
University of Torino – Department of Oncology Breast Cancer Associated (BRCA) gene 1 • Preclinical and retrospective studies initially suggested a predictive role for BRCA 1 with regard to cisplatin response 1 -5 • Phase III trial in stage IV NSCLC using BRCA 1 and RAP 80 expression levels showed a ↓ OS in the experimental arm compared to the control arm 6 • IALT - no association between BRCA 1 expression (IHC) and cisplatin response 7 1. Taron M et al. Hum Mol Genet 2004; 13; 2443 -9, 2. Papadaki C et al. J Thorac Oncol 2012; 7; 663 -71, 3. Rosell R et al, PLo. S ONE 2009; 4. e 5133, 4. Su C et al. Med Oncol 2001; 28; 1411 -7, 5. Bartolucci R et al, Clin Lung Cancer 2009; 10; 47 -52, 6. Moran T et al. J Clin Oncol, 2013; 31; LBA 8002, 7. Pierceall WE et al. Ann Oncol 2012; 23; 2245 -52

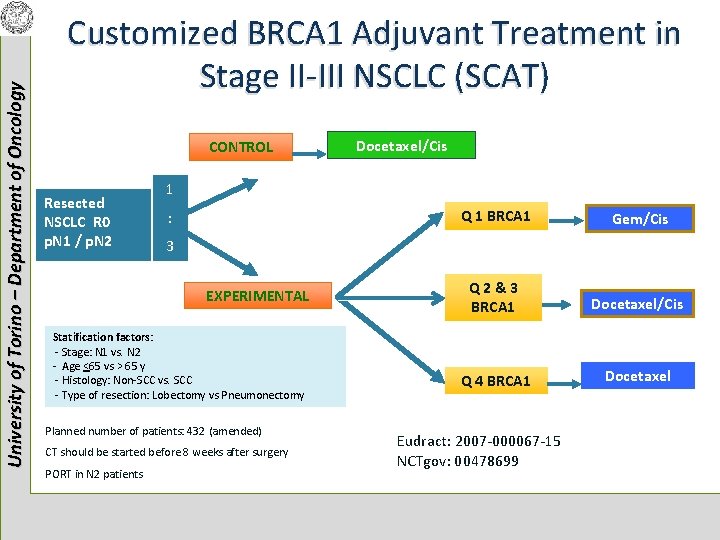
University of Torino – Department of Oncology Customized BRCA 1 Adjuvant Treatment in Stage II-III NSCLC (SCAT) CONTROL Resected NSCLC R 0 p. N 1 / p. N 2 1 : Q 1 BRCA 1 Gem/Cis Q 2&3 BRCA 1 Docetaxel/Cis Q 4 BRCA 1 Docetaxel 3 EXPERIMENTAL Statification factors: - Stage: N 1 vs. N 2 - Age <65 vs > 65 y - Histology: Non-SCC vs. SCC - Type of resection: Lobectomy vs Pneumonectomy Planned number of patients: 432 (amended) CT should be started before 8 weeks after surgery PORT in N 2 patients Docetaxel/Cis Eudract: 2007 -000067 -15 NCTgov: 00478699

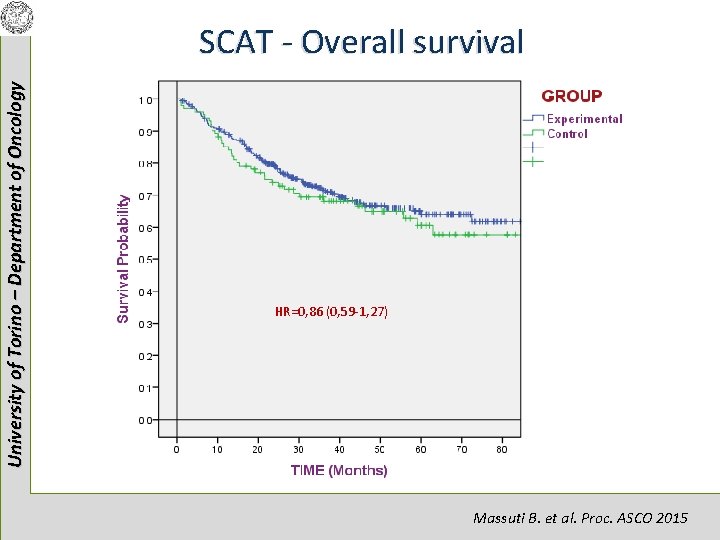
University of Torino – Department of Oncology SCAT - Overall survival HR=0, 86 (0, 59 -1, 27) Massuti B. et al. Proc. ASCO 2015

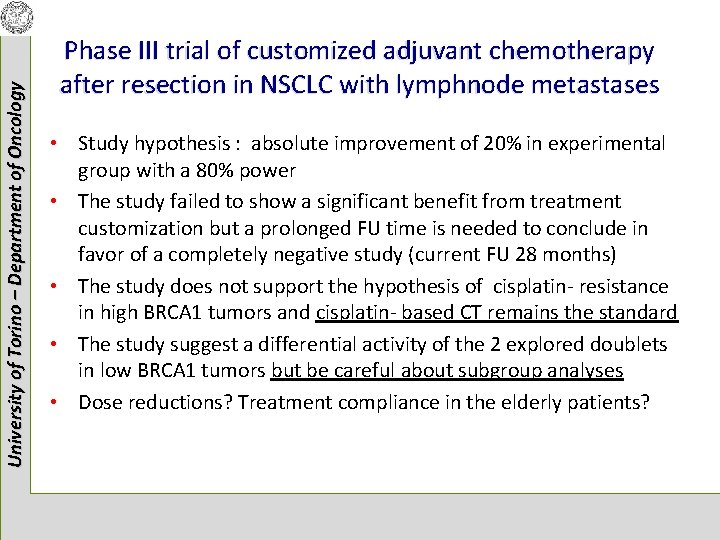
University of Torino – Department of Oncology Phase III trial of customized adjuvant chemotherapy after resection in NSCLC with lymphnode metastases • Study hypothesis : absolute improvement of 20% in experimental group with a 80% power • The study failed to show a significant benefit from treatment customization but a prolonged FU time is needed to conclude in favor of a completely negative study (current FU 28 months) • The study does not support the hypothesis of cisplatin- resistance in high BRCA 1 tumors and cisplatin- based CT remains the standard • The study suggest a differential activity of the 2 explored doublets in low BRCA 1 tumors but be careful about subgroup analyses • Dose reductions? Treatment compliance in the elderly patients?

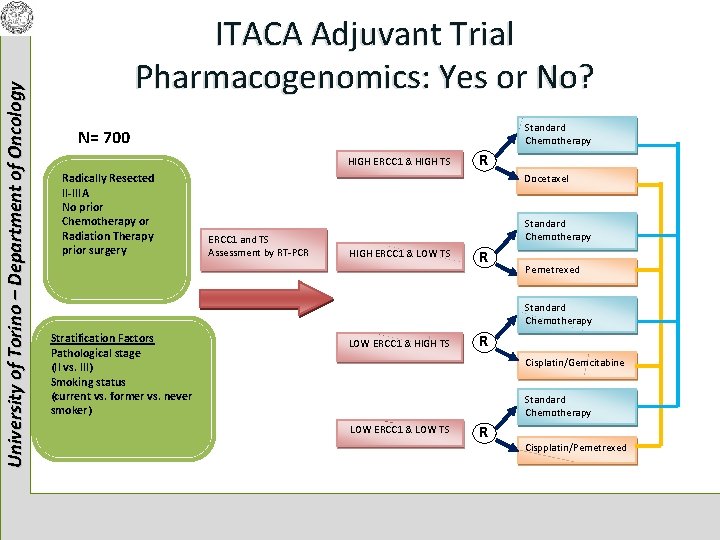
University of Torino – Department of Oncology ITACA Adjuvant Trial Pharmacogenomics: Yes or No? Standard Chemotherapy N= 700 HIGH ERCC 1 & HIGH TS Radically Resected II-IIIA No prior Chemotherapy or Radiation Therapy prior surgery R Docetaxel ERCC 1 and TS Assessment by RT-PCR Standard Chemotherapy HIGH ERCC 1 & LOW TS R Pemetrexed Standard Chemotherapy Stratification Factors Pathological stage (II vs. III) Smoking status (current vs. former vs. never smoker) LOW ERCC 1 & HIGH TS R Cisplatin/Gemcitabine Standard Chemotherapy LOW ERCC 1 & LOW TS R Cispplatin/Pemetrexed

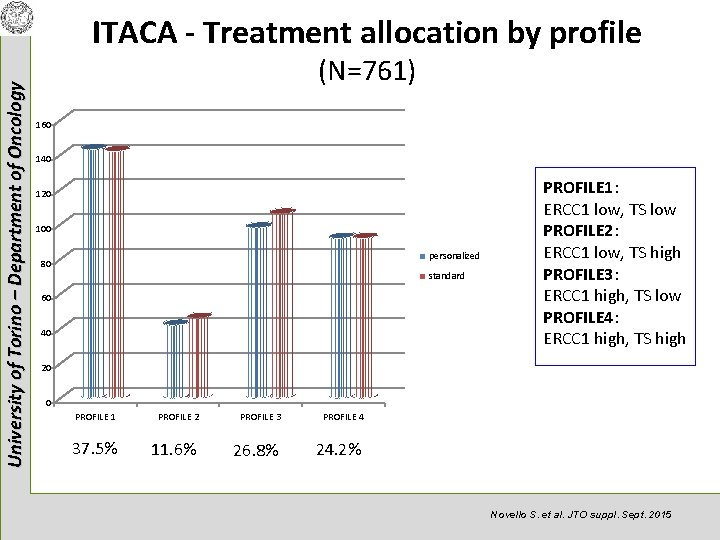
University of Torino – Department of Oncology ITACA - Treatment allocation by profile (N=761) 160 140 120 100 personalized 80 standard 60 40 PROFILE 1: ERCC 1 low, TS low PROFILE 2: ERCC 1 low, TS high PROFILE 3: ERCC 1 high, TS low PROFILE 4: ERCC 1 high, TS high 20 0 PROFILE 1 37. 5% PROFILE 2 11. 6% PROFILE 3 26. 8% PROFILE 4 24. 2% Novello S. et al. JTO suppl. Sept. 2015

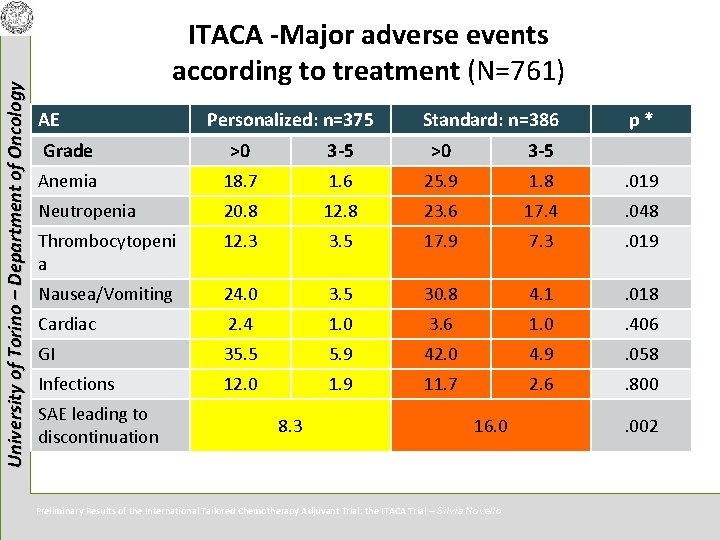
University of Torino – Department of Oncology ITACA -Major adverse events according to treatment (N=761) AE Personalized: n=375 Standard: n=386 p* Grade >0 3 -5 Anemia 18. 7 1. 6 25. 9 1. 8 . 019 Neutropenia 20. 8 12. 8 23. 6 17. 4 . 048 Thrombocytopeni a 12. 3 3. 5 17. 9 7. 3 . 019 Nausea/Vomiting 24. 0 3. 5 30. 8 4. 1 . 018 Cardiac 2. 4 1. 0 3. 6 1. 0 . 406 GI 35. 5 5. 9 42. 0 4. 9 . 058 Infections 12. 0 1. 9 11. 7 2. 6 . 800 SAE leading to discontinuation 8. 3 16. 0 Preliminary Results of the International Tailored Chemotherapy Adjuvant Trial: the ITACA Trial – Silvia Novello . 002

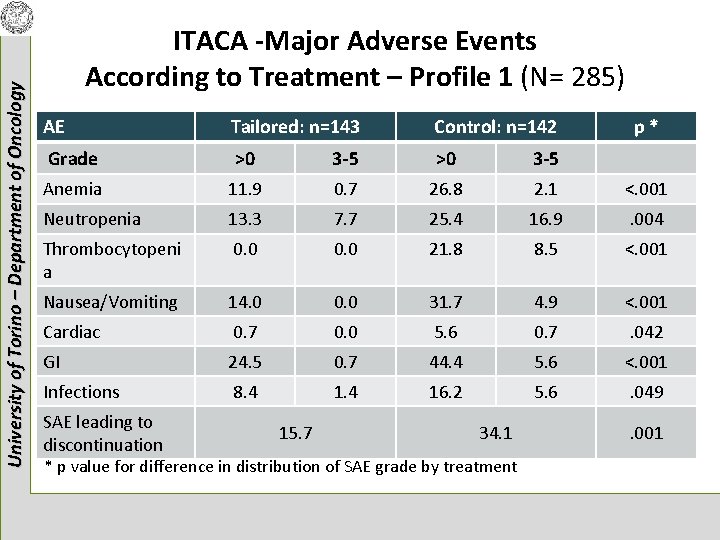
University of Torino – Department of Oncology ITACA -Major Adverse Events According to Treatment – Profile 1 (N= 285) AE Tailored: n=143 Control: n=142 Grade >0 3 -5 Anemia 11. 9 0. 7 26. 8 2. 1 <. 001 Neutropenia 13. 3 7. 7 25. 4 16. 9 . 004 Thrombocytopeni a 0. 0 21. 8 8. 5 <. 001 Nausea/Vomiting 14. 0 0. 0 31. 7 4. 9 <. 001 Cardiac 0. 7 0. 0 5. 6 0. 7 . 042 GI 24. 5 0. 7 44. 4 5. 6 <. 001 Infections 8. 4 16. 2 5. 6 . 049 SAE leading to discontinuation 15. 7 34. 1 * p value for difference in distribution of SAE grade by treatment p* . 001

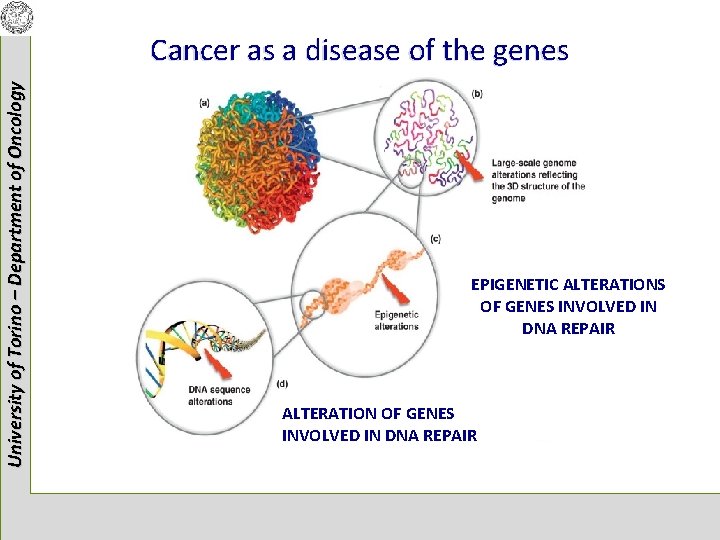
University of Torino – Department of Oncology Cancer as a disease of the genes EPIGENETIC ALTERATIONS OF GENES INVOLVED IN DNA REPAIR ALTERATION OF GENES INVOLVED IN DNA REPAIR

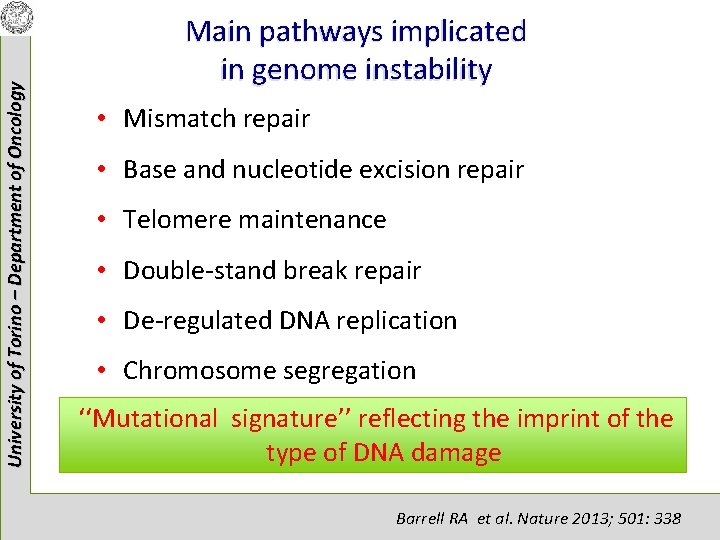
University of Torino – Department of Oncology Main pathways implicated in genome instability • Mismatch repair • Base and nucleotide excision repair • Telomere maintenance • Double-stand break repair • De-regulated DNA replication • Chromosome segregation ‘‘Mutational signature’’ reflecting the imprint of the type of DNA damage Barrell RA et al. Nature 2013; 501: 338

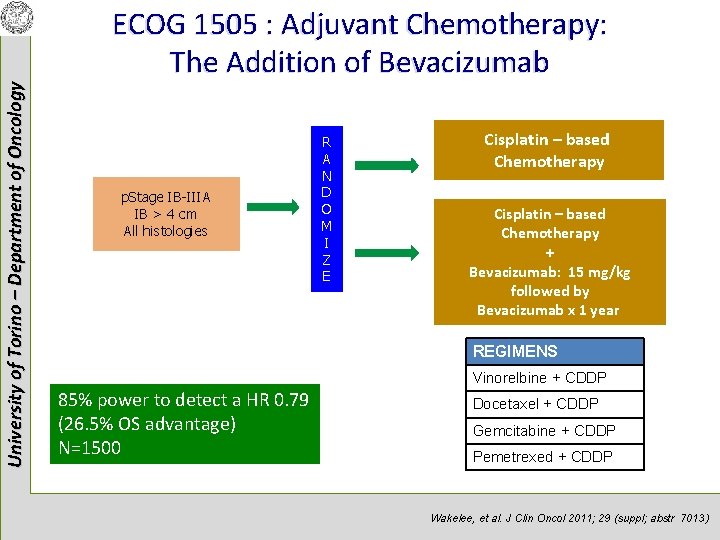
University of Torino – Department of Oncology ECOG 1505 : Adjuvant Chemotherapy: The Addition of Bevacizumab p. Stage IB-IIIA IB > 4 cm All histologies R A N D O M I Z E Cisplatin – based Chemotherapy + Bevacizumab: 15 mg/kg followed by Bevacizumab x 1 year REGIMENS Vinorelbine + CDDP 85% power to detect a HR 0. 79 (26. 5% OS advantage) N=1500 Docetaxel + CDDP Gemcitabine + CDDP Pemetrexed + CDDP Wakelee, et al. J Clin Oncol 2011; 29 (suppl; abstr 7013)

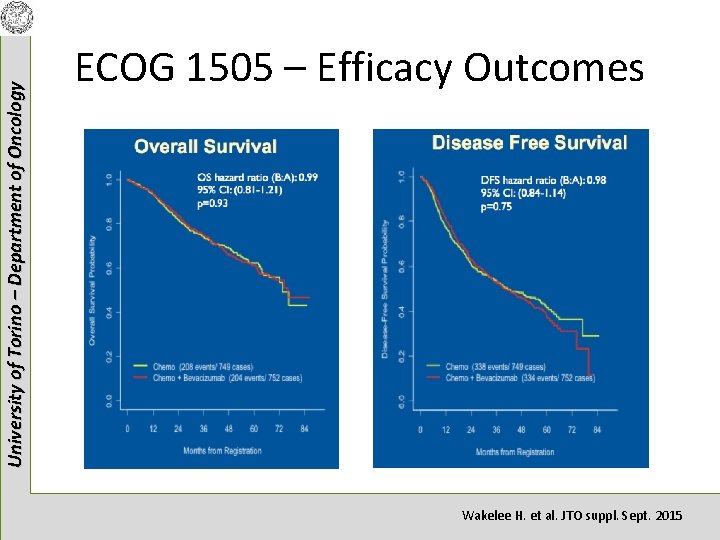
University of Torino – Department of Oncology ECOG 1505 – Efficacy Outcomes Wakelee H. et al. JTO suppl. Sept. 2015

University of Torino – Department of Oncology New Approaches to Adjuvant Therapy Can therapeutic advances that have been made with molecularly targeted therapy and immunotherapy in metastatic disease translate to increased cures in early stage disease?

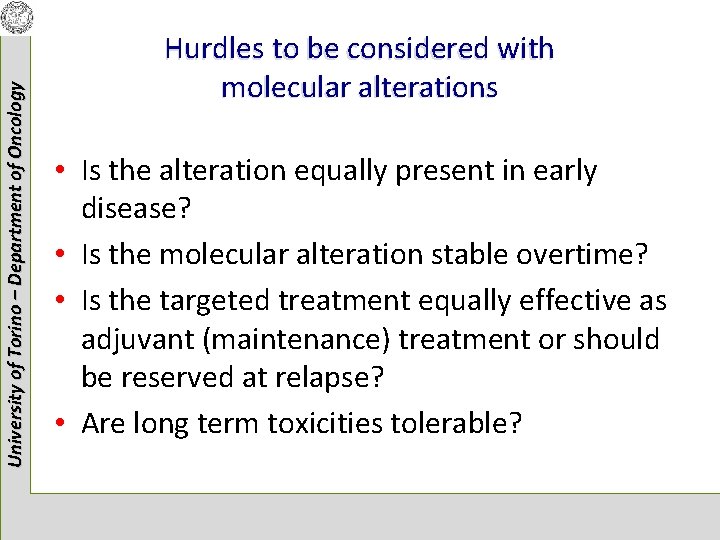
University of Torino – Department of Oncology Hurdles to be considered with molecular alterations • Is the alteration equally present in early disease? • Is the molecular alteration stable overtime? • Is the targeted treatment equally effective as adjuvant (maintenance) treatment or should be reserved at relapse? • Are long term toxicities tolerable?

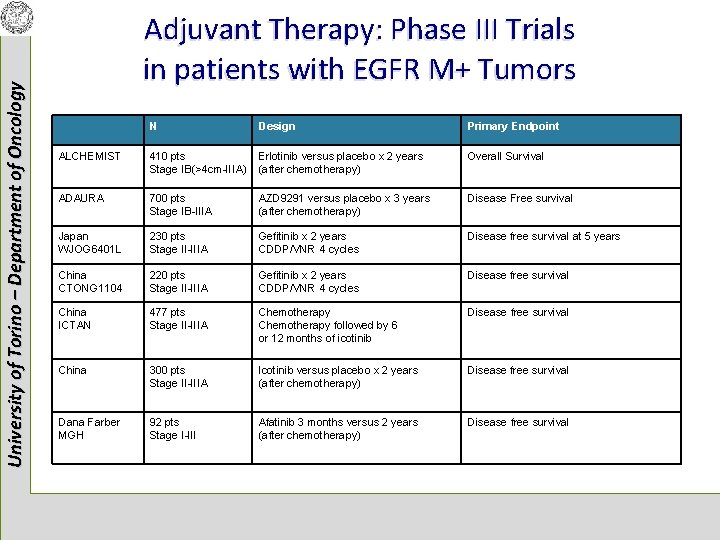
University of Torino – Department of Oncology Adjuvant Therapy: Phase III Trials in patients with EGFR M+ Tumors N Design Primary Endpoint ALCHEMIST 410 pts Stage IB(>4 cm-IIIA) Erlotinib versus placebo x 2 years (after chemotherapy) Overall Survival ADAURA 700 pts Stage IB-IIIA AZD 9291 versus placebo x 3 years (after chemotherapy) Disease Free survival Japan WJOG 6401 L 230 pts Stage II-IIIA Gefitinib x 2 years CDDP/VNR 4 cycles Disease free survival at 5 years China CTONG 1104 220 pts Stage II-IIIA Gefitinib x 2 years CDDP/VNR 4 cycles Disease free survival China ICTAN 477 pts Stage II-IIIA Chemotherapy followed by 6 or 12 months of icotinib Disease free survival China 300 pts Stage II-IIIA Icotinib versus placebo x 2 years (after chemotherapy) Disease free survival Dana Farber MGH 92 pts Stage I-III Afatinib 3 months versus 2 years (after chemotherapy) Disease free survival

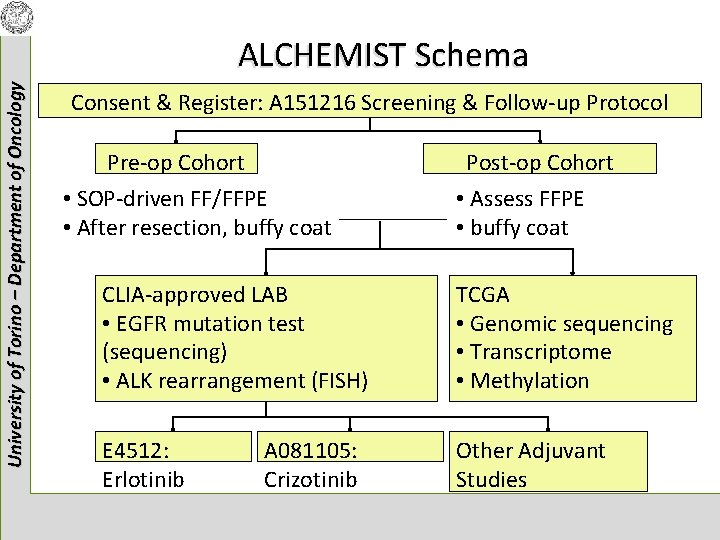
University of Torino – Department of Oncology ALCHEMIST Schema Consent & Register: A 151216 Screening & Follow-up Protocol Pre-op Cohort • SOP-driven FF/FFPE • After resection, buffy coat Post-op Cohort • Assess FFPE • buffy coat CLIA-approved LAB • EGFR mutation test (sequencing) • ALK rearrangement (FISH) TCGA • Genomic sequencing • Transcriptome • Methylation E 4512: Erlotinib Other Adjuvant Studies A 081105: Crizotinib

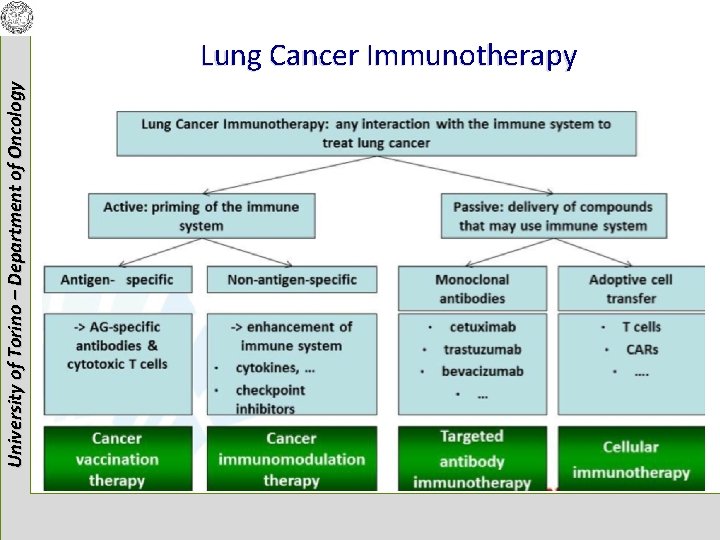
University of Torino – Department of Oncology Lung Cancer Immunotherapy

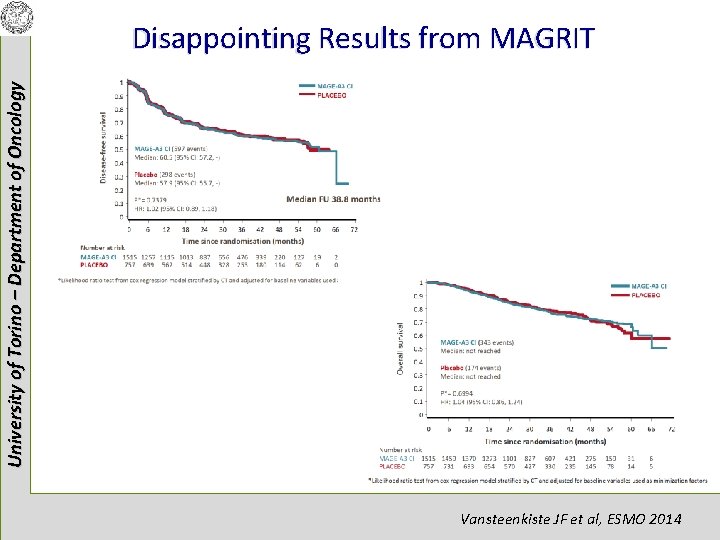
University of Torino – Department of Oncology Disappointing Results from MAGRIT Vansteenkiste JF et al, ESMO 2014

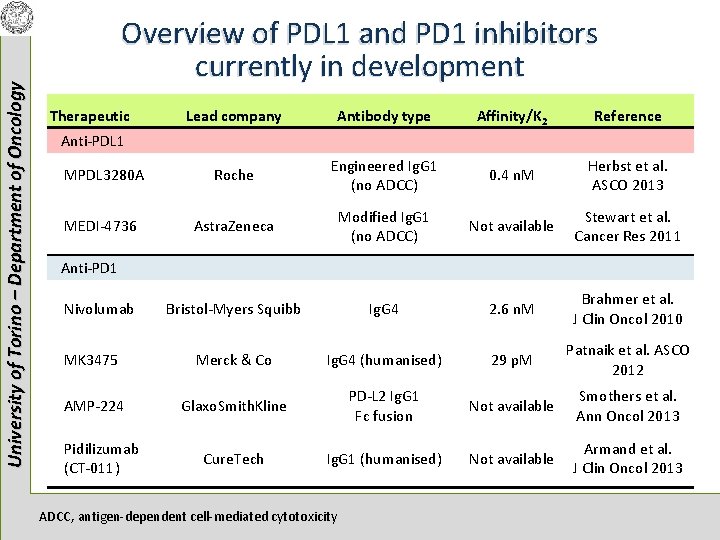
University of Torino – Department of Oncology Overview of PDL 1 and PD 1 inhibitors currently in development Therapeutic Lead company Antibody type Affinity/K 2 Reference MPDL 3280 A Roche Engineered Ig. G 1 (no ADCC) 0. 4 n. M Herbst et al. ASCO 2013 MEDI-4736 Astra. Zeneca Modified Ig. G 1 (no ADCC) Not available Stewart et al. Cancer Res 2011 Bristol-Myers Squibb Ig. G 4 2. 6 n. M Brahmer et al. J Clin Oncol 2010 MK 3475 Merck & Co Ig. G 4 (humanised) 29 p. M Patnaik et al. ASCO 2012 AMP-224 Glaxo. Smith. Kline PD-L 2 Ig. G 1 Fc fusion Not available Smothers et al. Ann Oncol 2013 Cure. Tech Ig. G 1 (humanised) Not available Armand et al. J Clin Oncol 2013 Anti-PDL 1 Anti-PD 1 Nivolumab Pidilizumab (CT-011) ADCC, antigen-dependent cell-mediated cytotoxicity

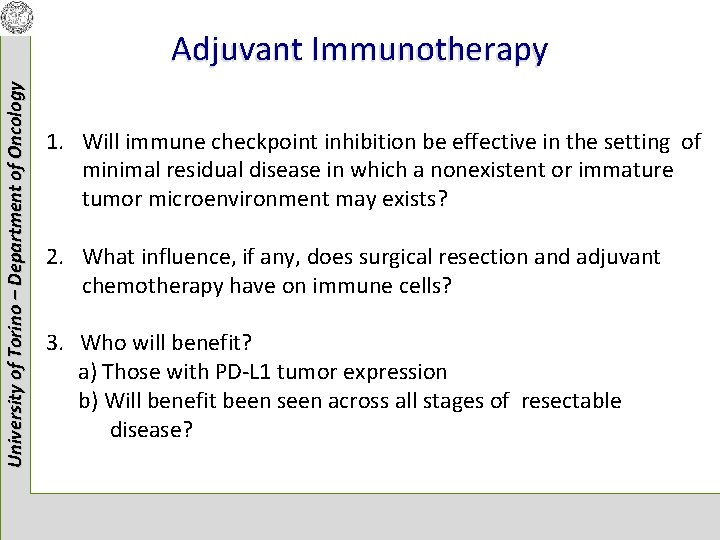
University of Torino – Department of Oncology Adjuvant Immunotherapy 1. Will immune checkpoint inhibition be effective in the setting of minimal residual disease in which a nonexistent or immature tumor microenvironment may exists? 2. What influence, if any, does surgical resection and adjuvant chemotherapy have on immune cells? 3. Who will benefit? a) Those with PD-L 1 tumor expression b) Will benefit been seen across all stages of resectable disease?

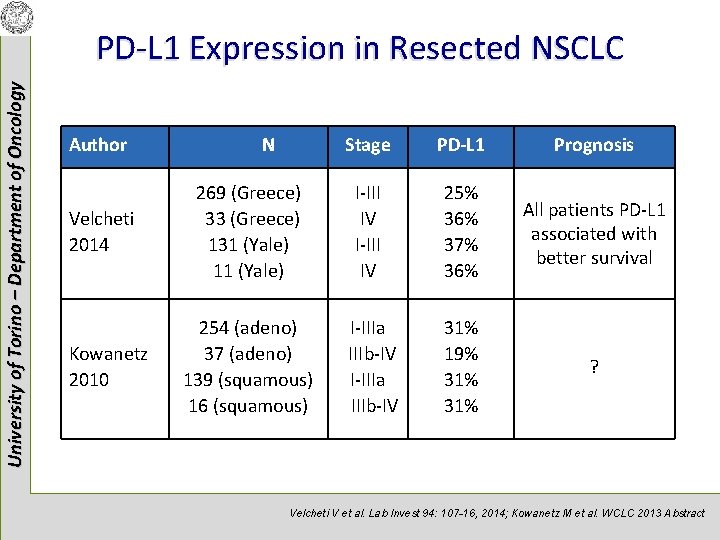
University of Torino – Department of Oncology PD-L 1 Expression in Resected NSCLC Author Velcheti 2014 Kowanetz 2010 N Stage PD-L 1 Prognosis 269 (Greece) 33 (Greece) 131 (Yale) 11 (Yale) I-III IV 25% 36% 37% 36% All patients PD-L 1 associated with better survival 254 (adeno) 37 (adeno) 139 (squamous) 16 (squamous) I-IIIa IIIb-IV 31% 19% 31% ? Velcheti V et al. Lab Invest 94: 107 -16, 2014; Kowanetz M et al. WCLC 2013 Abstract

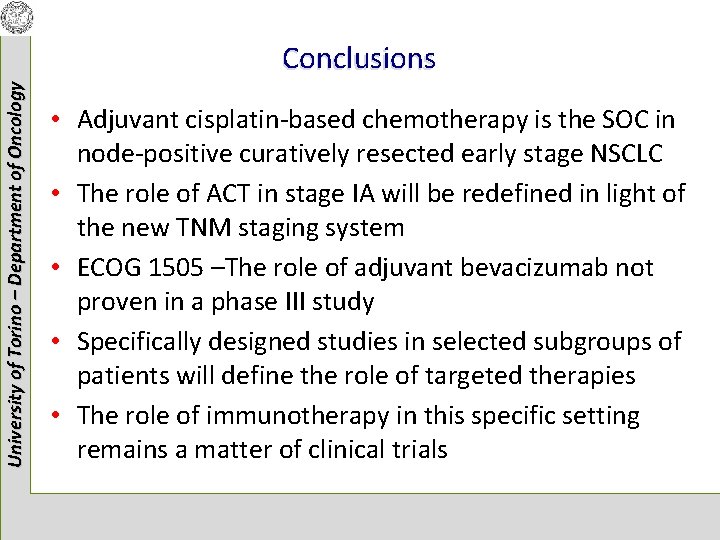
University of Torino – Department of Oncology Conclusions • Adjuvant cisplatin-based chemotherapy is the SOC in node-positive curatively resected early stage NSCLC • The role of ACT in stage IA will be redefined in light of the new TNM staging system • ECOG 1505 –The role of adjuvant bevacizumab not proven in a phase III study • Specifically designed studies in selected subgroups of patients will define the role of targeted therapies • The role of immunotherapy in this specific setting remains a matter of clinical trials
 Gondor adjuvant
Gondor adjuvant Adjuvant nsclc
Adjuvant nsclc Adjuvant neoadjuvant palliative
Adjuvant neoadjuvant palliative Adjuvant nsclc
Adjuvant nsclc Bmc oncology department
Bmc oncology department Unc chapel hill hematology oncology
Unc chapel hill hematology oncology Psychoanalytic vs humanistic
Psychoanalytic vs humanistic Bioness bits cost
Bioness bits cost Humanistic therapies aim to boost
Humanistic therapies aim to boost Cleveland state occupational therapy
Cleveland state occupational therapy University of florida occupational therapy
University of florida occupational therapy Grand valley state university occupational therapy
Grand valley state university occupational therapy [email protected]
[email protected] Department of law university of jammu
Department of law university of jammu Department of geology university of dhaka
Department of geology university of dhaka University of padova psychology
University of padova psychology University of bridgeport it department
University of bridgeport it department Iowa state math department
Iowa state math department Department of physics university of tokyo
Department of physics university of tokyo Texas state university psychology department
Texas state university psychology department Department of information engineering university of padova
Department of information engineering university of padova Information engineering padova
Information engineering padova Manipal university chemistry department
Manipal university chemistry department Syracuse university psychology department
Syracuse university psychology department Jackson state university finance department
Jackson state university finance department Columbia university department of computer science
Columbia university department of computer science Michigan state astronomy
Michigan state astronomy Columbia university cs department
Columbia university cs department University of sargodha engineering department
University of sargodha engineering department Stanford university philosophy department
Stanford university philosophy department Via castelgomberto 73
Via castelgomberto 73 Master plan
Master plan Nata a torino nel 1909
Nata a torino nel 1909 Borsellino torino residenza
Borsellino torino residenza Likkledet i torino
Likkledet i torino Ilo training centre
Ilo training centre Gran torino introduction
Gran torino introduction Carlo buffa neurologo torino
Carlo buffa neurologo torino Ilotte vincenzo
Ilotte vincenzo Ccfs unito
Ccfs unito Nadre ethiopia
Nadre ethiopia