Unit 4 Stoichiometry Chemical Equations and the Mole








- Slides: 16

Unit 4: Stoichiometry

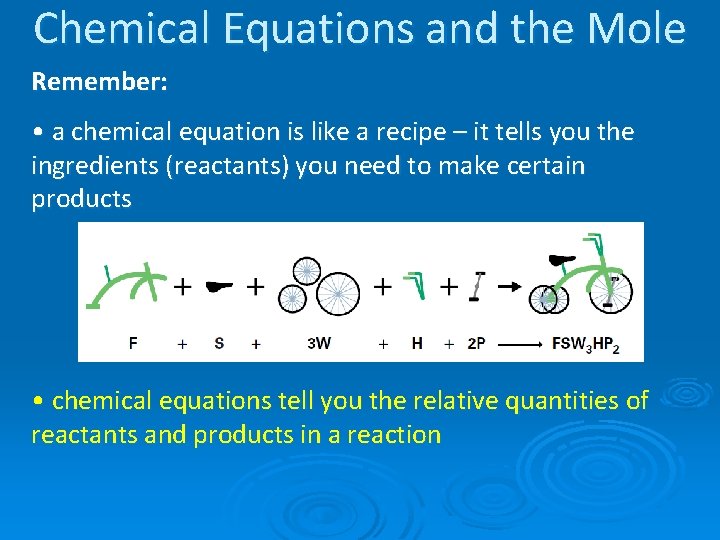
Chemical Equations and the Mole Remember: • a chemical equation is like a recipe – it tells you the ingredients (reactants) you need to make certain products • chemical equations tell you the relative quantities of reactants and products in a reaction

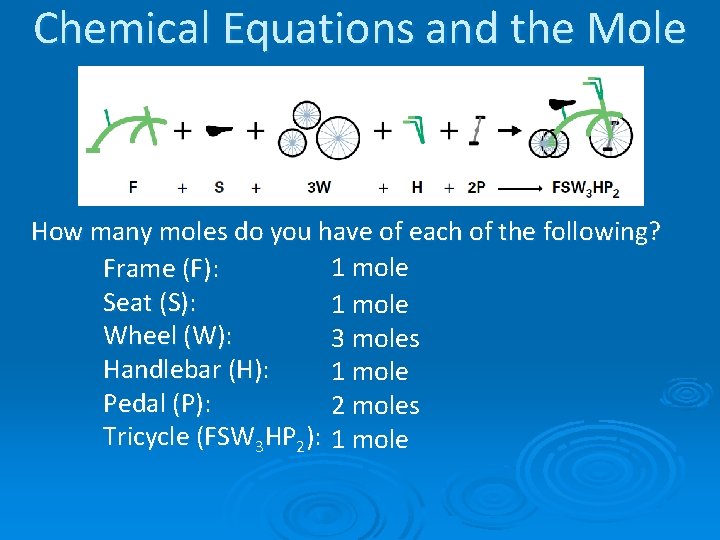
Chemical Equations and the Mole How many moles do you have of each of the following? 1 mole Frame (F): Seat (S): 1 mole Wheel (W): 3 moles Handlebar (H): 1 mole Pedal (P): 2 moles Tricycle (FSW 3 HP 2): 1 mole

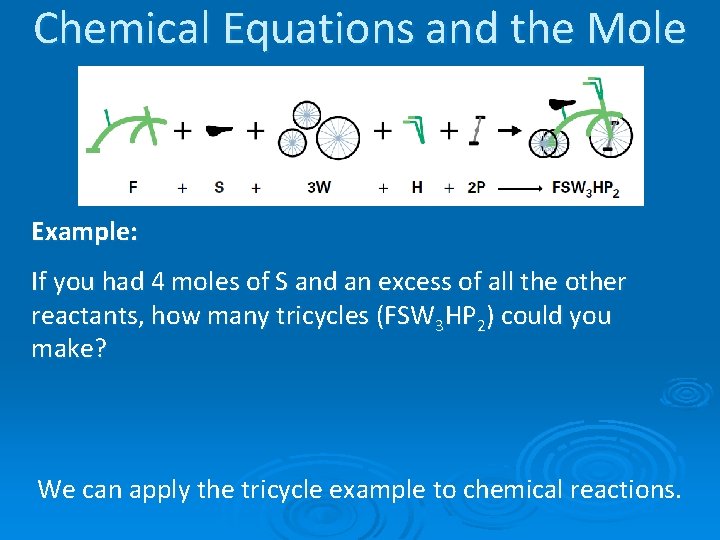
Chemical Equations and the Mole Example: If you had 4 moles of S and an excess of all the other reactants, how many tricycles (FSW 3 HP 2) could you make? We can apply the tricycle example to chemical reactions.

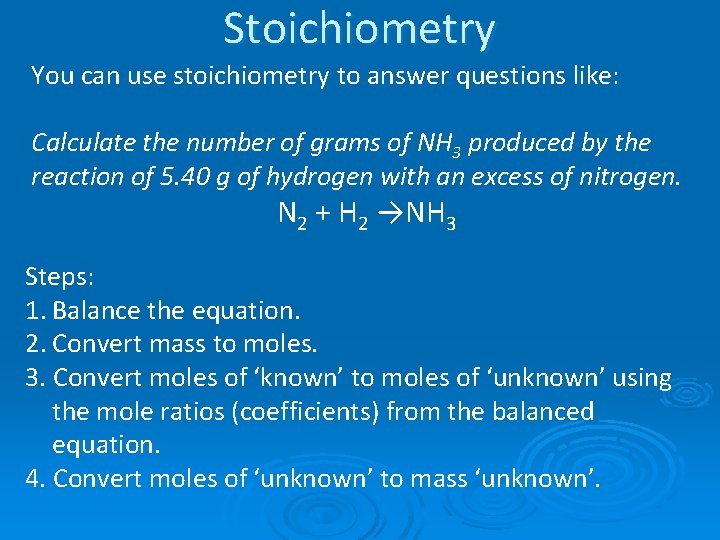
Stoichiometry: Using a known amount of one substance to predict how much of a reactant will be used or how much of a product will be produced. You can use stoichiometry to answer questions like: Calculate the number of grams of NH 3 produced by the reaction of 5. 40 g of nitrogen with an excess of hydrogen. N 2 + H 2 → NH 3

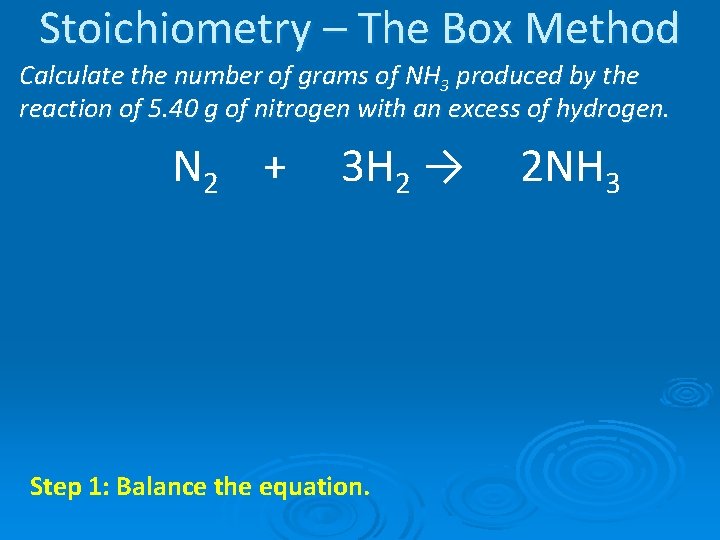
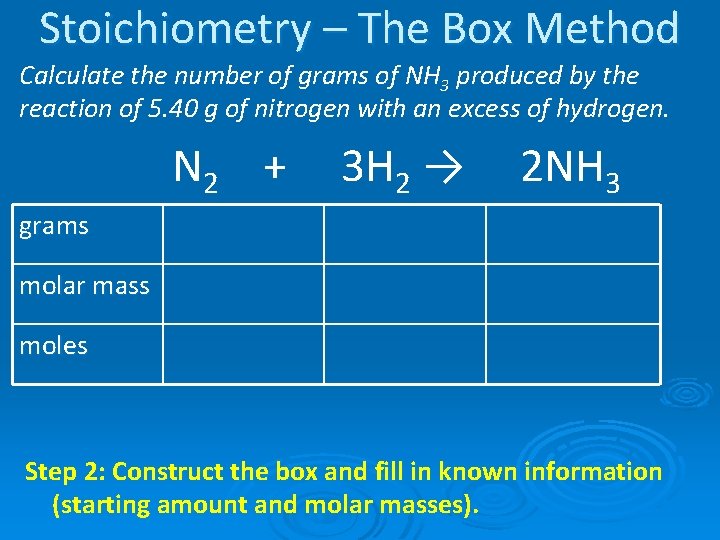
Stoichiometry – The Box Method Calculate the number of grams of NH 3 produced by the reaction of 5. 40 g of nitrogen with an excess of hydrogen. N 2 + 3 H 2 → Step 1: Balance the equation. 2 NH 3

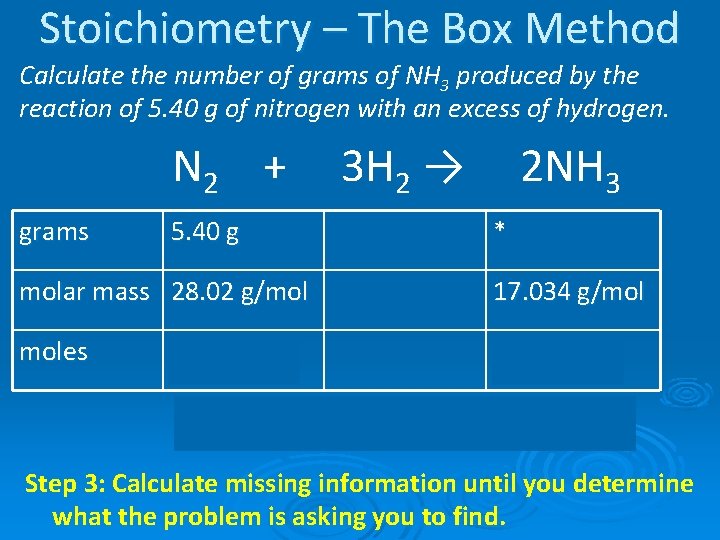
Stoichiometry – The Box Method Calculate the number of grams of NH 3 produced by the reaction of 5. 40 g of nitrogen with an excess of hydrogen. N 2 + grams 5. 40 g molar mass 28. 02 g/mol 3 H 2 → 2 NH 3 * 17. 034 g/mol moles Step 2: Construct the box and fill in known information (starting amount and molar masses).

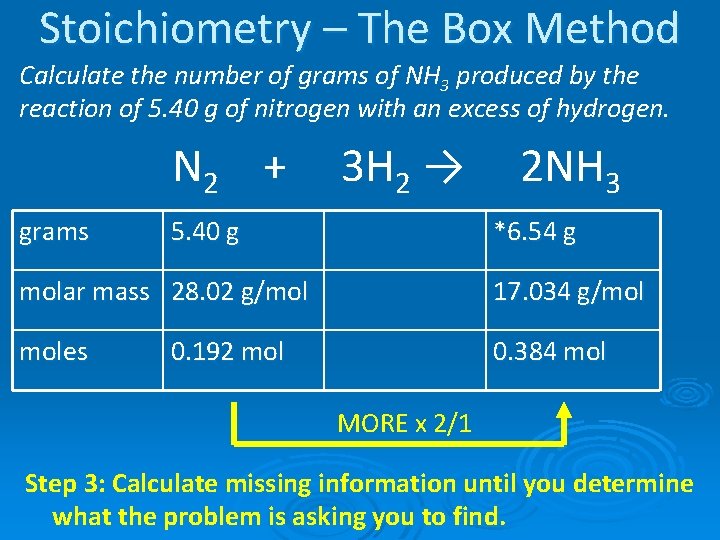
Stoichiometry – The Box Method Calculate the number of grams of NH 3 produced by the reaction of 5. 40 g of nitrogen with an excess of hydrogen. N 2 + grams 3 H 2 → 5. 40 g 2 NH 3 *6. 54 g molar mass 28. 02 g/mol 17. 034 g/mol moles 0. 384 mol 0. 192 mol MORE x 2/1 Step 3: Calculate missing information until you determine what the problem is asking you to find.

Stoichiometry – The Box Method Calculate the number of grams of NH 3 produced by the reaction of 5. 40 g of nitrogen with an excess of hydrogen. N 2 + grams 3 H 2 → 5. 40 g 2 NH 3 *6. 54 g molar mass 28. 02 g/mol 17. 034 g/mol moles 0. 384 mol 0. 192 mol MORE x 2/1 Step 3: Calculate missing information until you determine what the problem is asking you to find.

Stoichiometry – Practice! How many grams of water would be produced from 60 grams of H 2 and an excess of O 2? __H 2 + __O 2 → __H 2 O


Stoichiometry You can use stoichiometry to answer questions like: Calculate the number of grams of NH 3 produced by the reaction of 5. 40 g of hydrogen with an excess of nitrogen. N 2 + H 2 →NH 3 Steps: 1. Balance the equation. 2. Convert mass to moles. 3. Convert moles of ‘known’ to moles of ‘unknown’ using the mole ratios (coefficients) from the balanced equation. 4. Convert moles of ‘unknown’ to mass ‘unknown’.

Stoichiometry – Step 1 Calculate the number of grams of NH 3 produced by the reaction of 5. 40 g of hydrogen with an excess of nitrogen. N 2 + H 2 →NH 3 Step 1: Balance the equation.

Stoichiometry – Step 2 Calculate the number of grams of NH 3 produced by the reaction of 5. 40 g of hydrogen with an excess of nitrogen. N 2 + H 2 →NH 3 Step 2: Convert mass to moles.

Stoichiometry – Step 3 Calculate the number of grams of NH 3 produced by the reaction of 5. 40 g of hydrogen with an excess of nitrogen. N 2 + H 2 → NH 3 Step 3: Convert moles of ‘known’ to moles of ‘unknown’ using the mole ratios (coefficients) from the balanced equation.

Stoichiometry – Step 4 Calculate the number of grams of NH 3 produced by the reaction of 5. 40 g of hydrogen with an excess of nitrogen. N 2 + H 2 →NH 3 Step 4: Convert moles of ‘unknown’ to mass ‘unknown’.