Types of Chemical Reactions 12192021 Dr Seemal Jelani



- Slides: 25

Types of Chemical Reactions 12/19/2021 Dr Seemal Jelani Chem-100 1

Learning Outcomes Identify, give evidence for, predict products of, and classify the following types of chemical reactions: 1. Synthesis (combination) 2. Decomposition 3. Single Replacement 4. Double Replacement 5. Neutralization (acid/base) 6. Combustion 12/19/2021 Dr Seemal Jelani Chem-100 2

Vocabulary q Synthesis q Decomposition q Neutralization q Single displacement q Double displacement q Combustion 12/19/2021 Dr Seemal Jelani Chem-100 3

Chemical Reactions A chemical change: any change in which a new substance is formed. Evidence of a Chemical Change: v Release of energy as heat v Release of energy as light v Change in colour v Formation of a gas v Change in odour… 12/19/2021 Dr Seemal Jelani Chem-100 4

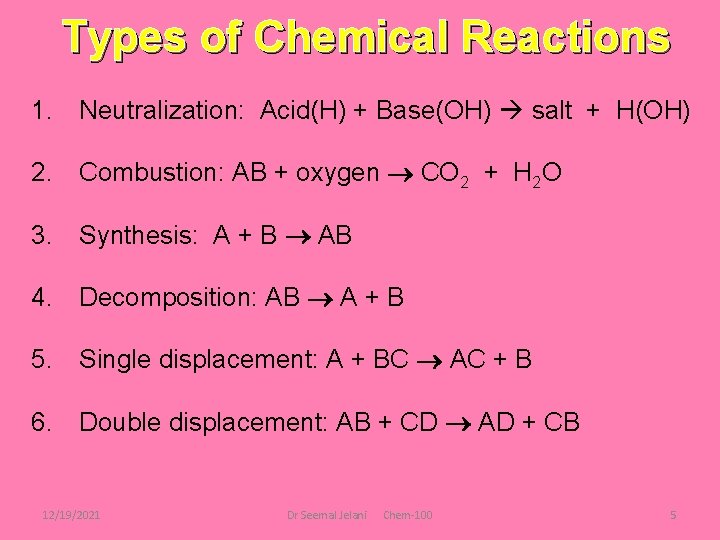
Types of Chemical Reactions 1. Neutralization: Acid(H) + Base(OH) salt + H(OH) 2. Combustion: AB + oxygen CO 2 + H 2 O 3. Synthesis: A + B AB 4. Decomposition: AB A + B 5. Single displacement: A + BC AC + B 6. Double displacement: AB + CD AD + CB 12/19/2021 Dr Seemal Jelani Chem-100 5

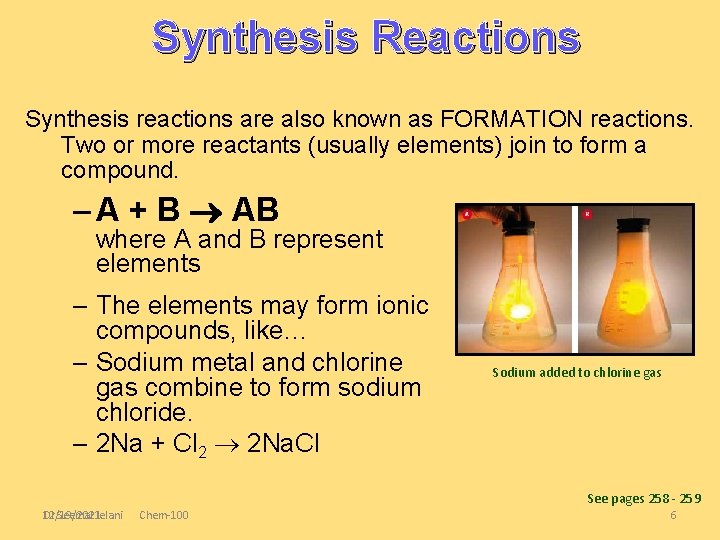
Synthesis Reactions Synthesis reactions are also known as FORMATION reactions. Two or more reactants (usually elements) join to form a compound. – A + B AB where A and B represent elements – The elements may form ionic compounds, like… – Sodium metal and chlorine gas combine to form sodium chloride. – 2 Na + Cl 2 2 Na. Cl Sodium added to chlorine gas See pages 258 - 259 Dr Seemal Jelani 12/19/2021 Chem-100 6

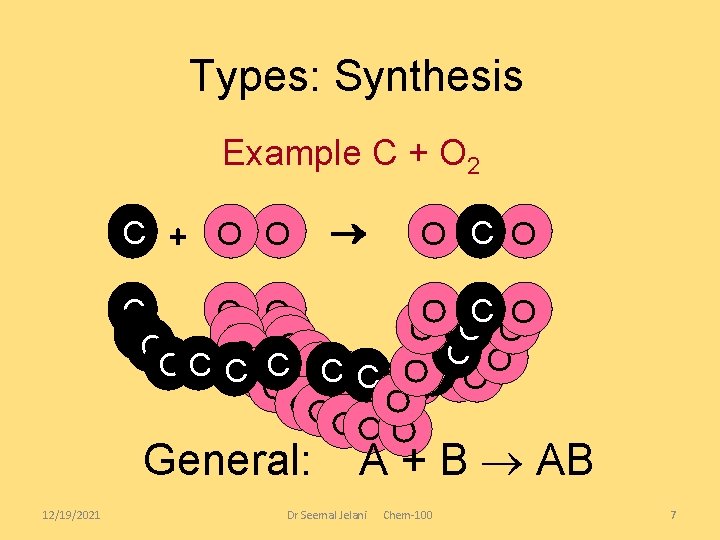
Types: Synthesis Example C + O 2 C + O O O C O CC O O O C C C OC OOC C OCO OO CO O O OO OO OOO General: 12/19/2021 A + B AB Dr Seemal Jelani Chem-100 7

Synthesis Reactions OTHER EXAMPLES… 1. Elements that form ionic compounds: Magnesium metal reacts with oxygen gas to form magnesium oxide. • 2 Mg + O 2 2 Mg. O 2. Elements that form covalent compounds: Nitrogen gas and oxygen gas join to form dinitrogen monoxide. • 2 N 2 + O 2 2 N 2 O SYNTHESIS REACTION (iron + sulphur): http: //www. youtube. com/watch? v=A 5 H 6 DVe 5 FAI Dr Seemal Jelani 12/19/2021 Chem-100 See pages 258 - 259 8

Decomposition Reactions • Decomposition reactions are the opposite of synthesis reactions. – A compounds breaks down into two or more products (often elements). – AB A + B where A and B represent elements 1. Ionic compounds may decompose to produce elements, like the following: • Table salt, sodium chloride, can be broken down into sodium metal and chlorine gas by melting salt at 800ºC and running electricity through it. • 2 Na. Cl 2 Na + Cl 2 See page 260 Dr Seemal Jelani 12/19/2021 Chem-100 9

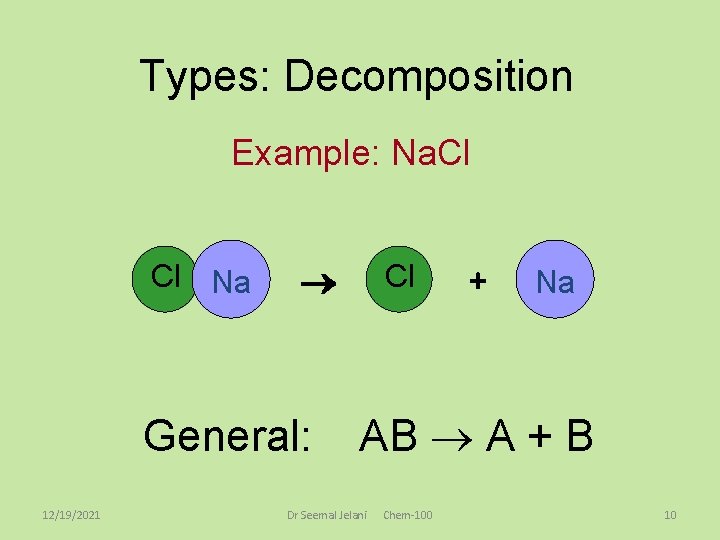
Types: Decomposition Example: Na. Cl Cl Na General: 12/19/2021 Cl + Na AB A + B Dr Seemal Jelani Chem-100 10

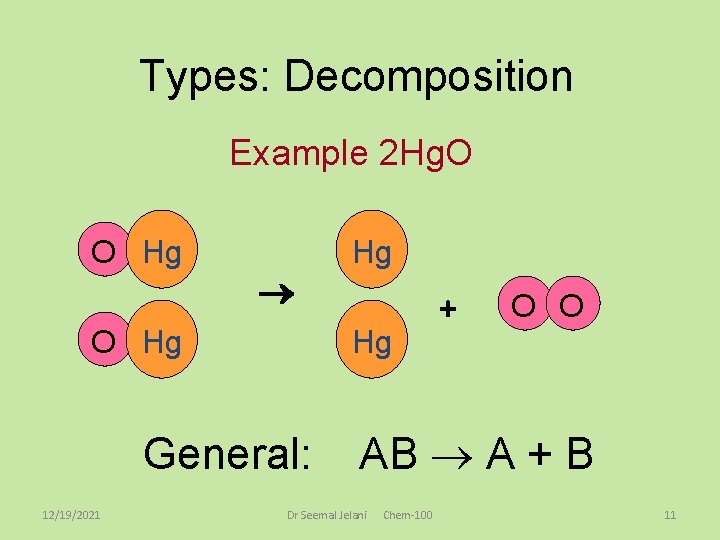
Types: Decomposition Example 2 Hg. O O Hg Hg O Hg General: 12/19/2021 Hg + O O AB A + B Dr Seemal Jelani Chem-100 11

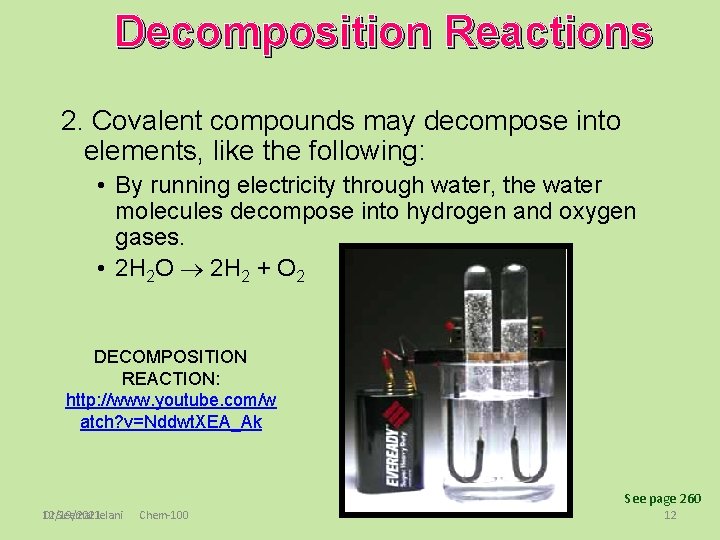
Decomposition Reactions 2. Covalent compounds may decompose into elements, like the following: • By running electricity through water, the water molecules decompose into hydrogen and oxygen gases. • 2 H 2 O 2 H 2 + O 2 DECOMPOSITION REACTION: http: //www. youtube. com/w atch? v=Nddwt. XEA_Ak See page 260 Dr Seemal Jelani 12/19/2021 Chem-100 12

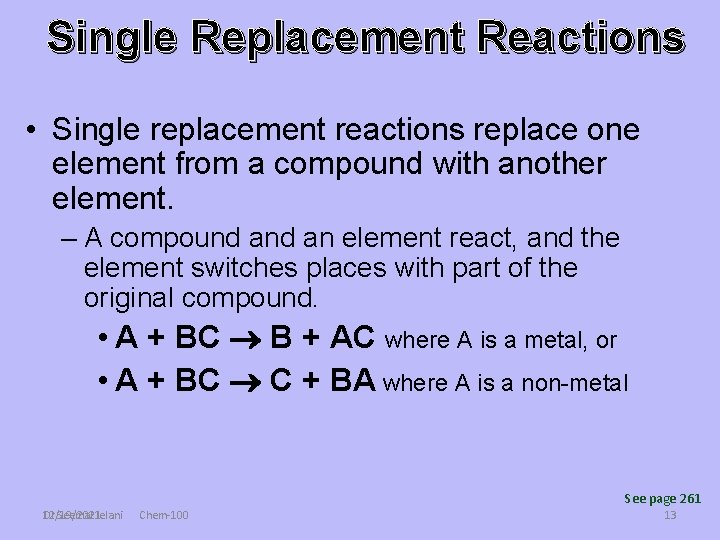
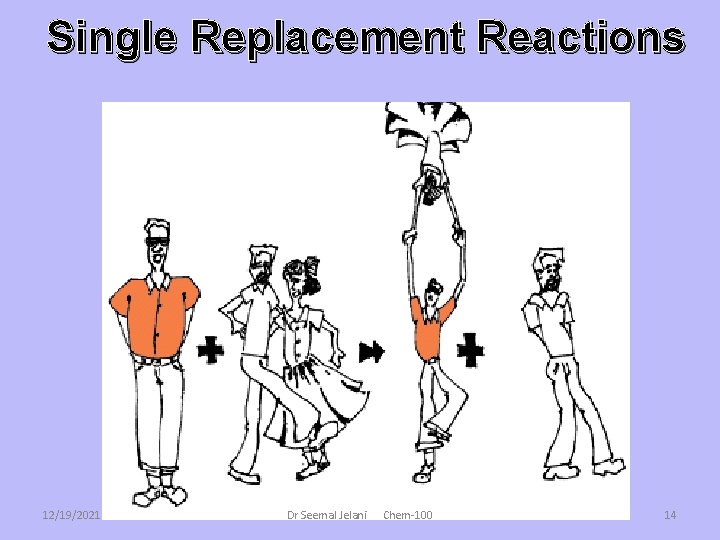
Single Replacement Reactions • Single replacement reactions replace one element from a compound with another element. – A compound an element react, and the element switches places with part of the original compound. • A + BC B + AC where A is a metal, or • A + BC C + BA where A is a non-metal See page 261 Dr Seemal Jelani 12/19/2021 Chem-100 13

Single Replacement Reactions 12/19/2021 Dr Seemal Jelani Chem-100 14

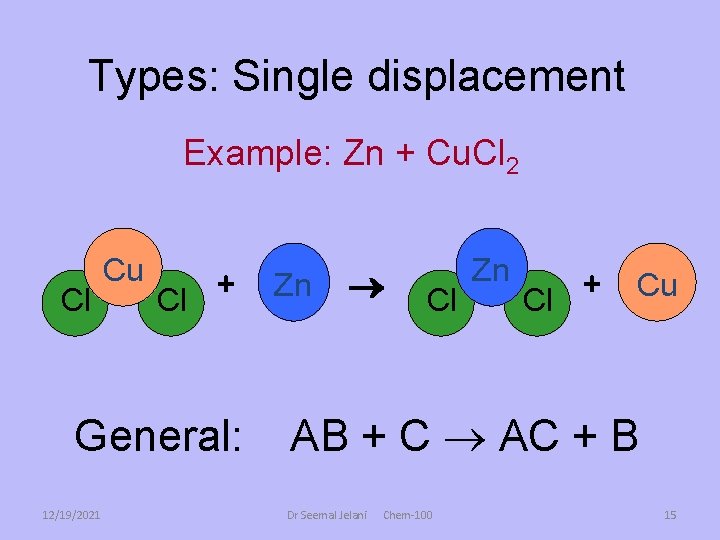
Types: Single displacement Example: Zn + Cu. Cl 2 Cl Cu + Cl General: 12/19/2021 Zn Cl Zn + Cu Cl AB + C AC + B Dr Seemal Jelani Chem-100 15

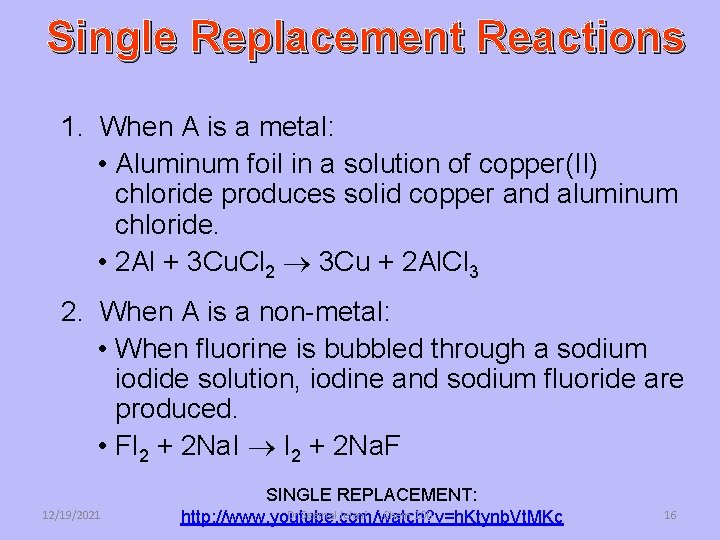
Single Replacement Reactions 1. When A is a metal: • Aluminum foil in a solution of copper(II) chloride produces solid copper and aluminum chloride. • 2 Al + 3 Cu. Cl 2 3 Cu + 2 Al. Cl 3 2. When A is a non-metal: • When fluorine is bubbled through a sodium iodide solution, iodine and sodium fluoride are produced. • Fl 2 + 2 Na. I I 2 + 2 Na. F 12/19/2021 SINGLE REPLACEMENT: Dr Seemal Jelani Chem-100 http: //www. youtube. com/watch? v=h. Ktynb. Vt. MKc 16

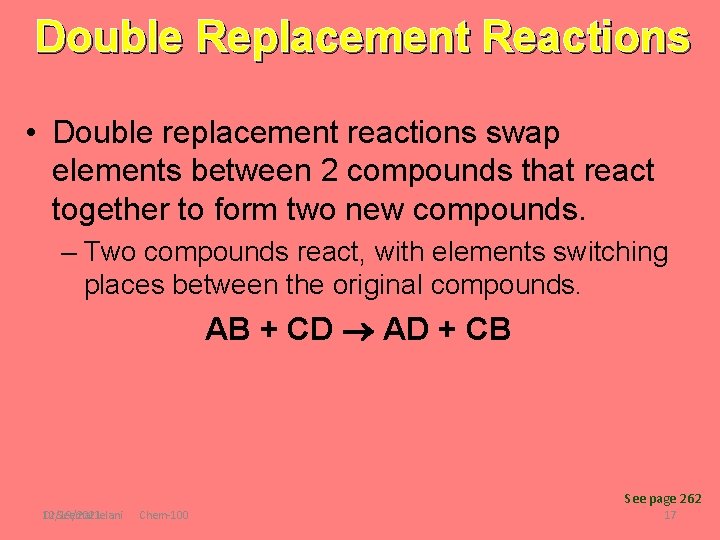
Double Replacement Reactions • Double replacement reactions swap elements between 2 compounds that react together to form two new compounds. – Two compounds react, with elements switching places between the original compounds. AB + CD AD + CB See page 262 Dr Seemal Jelani 12/19/2021 Chem-100 17

Double Replacement Reactions See page 262 Dr Seemal Jelani 12/19/2021 Chem-100 18

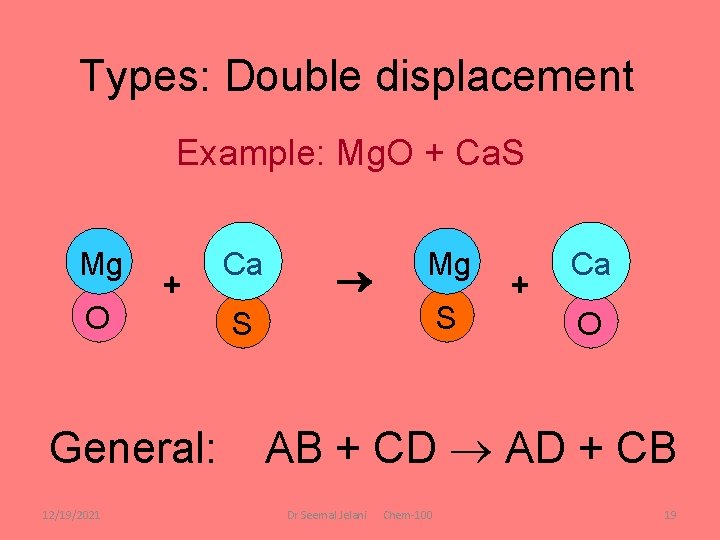
Types: Double displacement Example: Mg. O + Ca. S Mg O + General: 12/19/2021 Ca Mg S S + Ca O AB + CD AD + CB Dr Seemal Jelani Chem-100 19

Double Replacement Reactions Two solutions react to form a precipitate (solid) and another solution. Ionic solution + ionic solid. AB + CD AD + CB – When potassium chromate and silver nitrate react, they form a red precipitate, silver chromate, in a solution of potassium nitrate. – K 2 Cr. O 4 + 2 Ag. NO 3 Ag 2 Cr. O 4 + 2 KNO 3 Dr Seemal Jelani 12/19/2021 silver chromate DOUBLE REPLACEMENT: http: //www. youtube. com/watch? v=op. Y 3 FLr. PTa 4 Chem-100 20

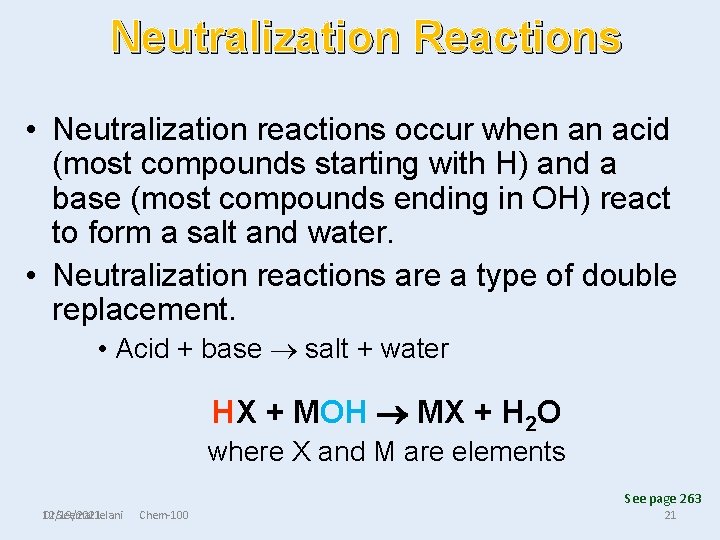
Neutralization Reactions • Neutralization reactions occur when an acid (most compounds starting with H) and a base (most compounds ending in OH) react to form a salt and water. • Neutralization reactions are a type of double replacement. • Acid + base salt + water HX + MOH MX + H 2 O where X and M are elements See page 263 Dr Seemal Jelani 12/19/2021 Chem-100 21

Neutralization Reactions 1. Sulfuric acid is used to neutralize calcium hydroxide: • H 2 SO 4 + Ca(OH) 2 Ca. SO 4 + 2 H 2 O 2. Phosphoric acid helps to neutralize the compounds that cause rust, such as iron(II) hydroxide. • H 3 PO 4 + 3 Fe(OH)2 Fe 3(PO 4)2 + 6 H 2 O http: //www. youtube. com/watch? v=_P 5 h. Gz. A 6 Vb 0 See page 263 Dr Seemal Jelani 12/19/2021 Chem-100 22

Combustion Reactions • Combustion reactions occur when a compound or element react with oxygen to release energy and produce an oxide. – Also sometimes referred to as hydrocarbon combustion. CXHY + O 2 CO 2 + H 2 O where X and Y represent integers METHANOL + oxygen: http: //www. youtube. com/watch? v=98 Ju. J-G 1 q. XY&feature=related See page 264 Dr Seemal Jelani 12/19/2021 Chem-100 23

Combustion Reactions 1. Natural gas (methane) is burned in furnaces to heat homes. » CH 4 + O 2 CO 2 + 2 H 2 O + energy 2. An acetylene torch is used to weld metals together. » 2 C 2 H 2 + 5 O 2 4 CO 2 + 2 H 2 O + energy 3. Carbohydrates like glucose combine with oxygen in our body to release energy. Acetylene torch » C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O + energy See page 264 Dr Seemal Jelani 12/19/2021 Chem-100 24

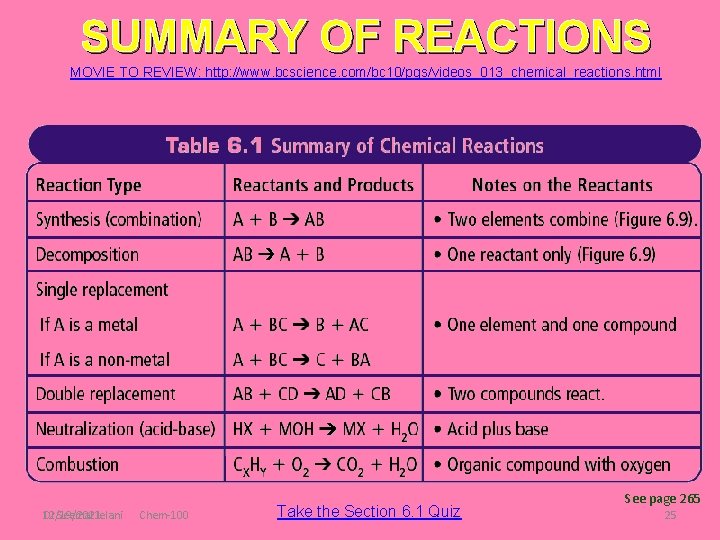
SUMMARY OF REACTIONS MOVIE TO REVIEW: http: //www. bcscience. com/bc 10/pgs/videos_013_chemical_reactions. html Dr Seemal Jelani 12/19/2021 Chem-100 Take the Section 6. 1 Quiz See page 265 25
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Seemal desai
Seemal desai Types of reactions
Types of reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Types of redox reactions
Types of redox reactions Identify types of reactions
Identify types of reactions Types of reactions chemistry
Types of reactions chemistry Chemical reaction types
Chemical reaction types 4 types of chemical reactions
4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions 5 general types of chemical reactions
5 general types of chemical reactions 5 general types of chemical reactions
5 general types of chemical reactions What are the 4 types of chemical reactions
What are the 4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions What is the type of reaction
What is the type of reaction Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Fatoumata dembele chef
Fatoumata dembele chef Combustion reaction
Combustion reaction Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry 20 examples of redox reaction
20 examples of redox reaction Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Stoichiometry mole island diagram
Stoichiometry mole island diagram Balancing chemical equations definition
Balancing chemical equations definition Predicting products of chemical reactions
Predicting products of chemical reactions