Training for the introduction of Inactivated Poliovirus Vaccine













- Slides: 22

Training for the introduction of Inactivated Poliovirus Vaccine, Fractional Dose (f. IPV) Module 4 Fractional IPV (f. IPV) vaccine administration

Learning objectives l At the end of the module, the participant will be able to: – Identify the necessary steps to assure good vaccine quality – Describe the method to administer the vaccine l Duration – 30 minutes 2| IPV vaccine administration, Module 4 | October 2020

Key issues 1 How do I check vaccine quality? 2 How do I prepare for vaccination? 3 How do I administer the vaccine? 4 How do I administer IPV at the same time as other routine immunizations? 3| IPV vaccine administration, Module 4 | October 2020

IPV is heat and freeze sensitive l IPV loses potency when exposed to heat or when frozen – Store at +2°C to +8°C Warming vaccines shortens shelf life l IPV is freeze sensitive – Unlike OPV, which can be frozen – The “shake test” is ineffective in determining whether IPV has been frozen – If you suspect that IPV may have been frozen, the vial must be discarded Aim for 4⁰-5⁰C Freezing KILLS vaccines! Except OPV, Vaccines that have been frozen are ineffective l Do not use if vaccine has a cloudy appearance l Check the VVM and the expiration date (see next 2 slides) 4| IPV vaccine administration, Module 4 | October 2020

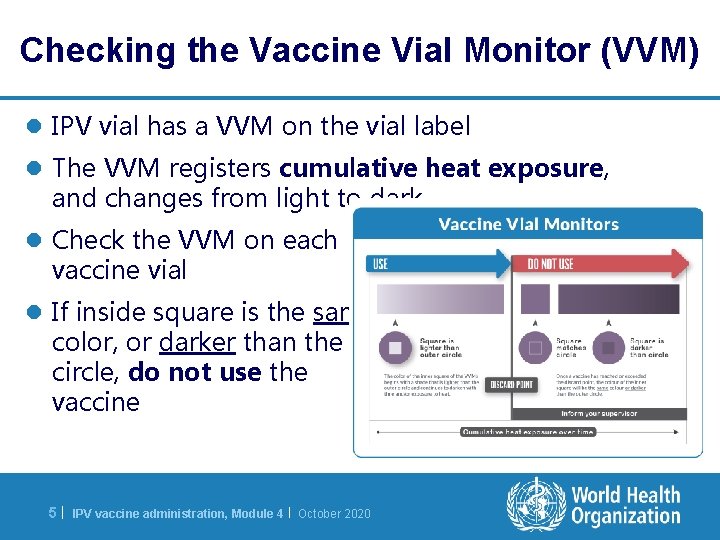
Checking the Vaccine Vial Monitor (VVM) l IPV vial has a VVM on the vial label l The VVM registers cumulative heat exposure, and changes from light to dark l Check the VVM on each vaccine vial l If inside square is the same color, or darker than the circle, do not use the vaccine 5| IPV vaccine administration, Module 4 | October 2020

IPV has high heat sensitivity l IPV has increased susceptibility to heat than many existing heat sensitive vaccines l VVM on IPV may change color faster than other vaccines l Proper temperature monitoring and stock management is required to avoid wasting IPV vials with VVM reaching the discard point l While the “earliest expiry, first out” principal usually applies in vaccine stock management, the status of a VVM overrules this, whereby any batch showing a darker VVM should be used sooner, regardless of a later expiry date 6 | IPV vaccine administration, Module 4 | October 2020

Checking the expiration date l Vaccine loses potency over time l VVM provides information about storage conditions, but not about potency l VVM may be OK, but vaccine may be expired l Before administering any vaccine, always check the expiration date • Expiration date: 02 NOV 14 • Use through November 2, 2014 • Do NOT use on or after November 3, 2014 7| IPV vaccine administration, Module 4 | October 2020

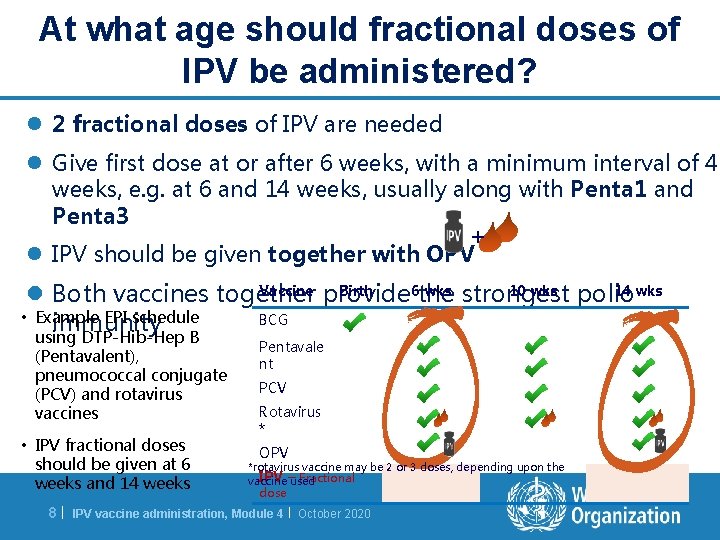
At what age should fractional doses of IPV be administered? l 2 fractional doses of IPV are needed l Give first dose at or after 6 weeks, with a minimum interval of 4 weeks, e. g. at 6 and 14 weeks, usually along with Penta 1 and Penta 3 + l IPV should be given together with OPV Vaccine provide Birth 6 wks 10 wks polio 14 wks l Both vaccines together the strongest • Example EPI schedule BCG immunity using DTP-Hib-Hep B (Pentavalent), pneumococcal conjugate (PCV) and rotavirus vaccines • IPV fractional doses should be given at 6 weeks and 14 weeks 8| Pentavale nt PCV Rotavirus * OPV *rotavirus vaccine may be 2 or 3 doses, depending upon the IPV –used Fractional vaccine dose IPV vaccine administration, Module 4 | October 2020

How to prepare for vaccination l Prepare IPV at the same time you prepare other vaccines l IPV can be administered with any of the following routine childhood vaccines without interfering with their effectiveness: l – Diphtheria–tetanus–pertussis vaccine (DTP)/pentavalent vaccine – Haemophilus influenzae type b vaccine (Hib) – Pneumococcal vaccine – Oral polio vaccine (OPV) Never mix IPV with other vaccines in the same vial or – Rotavirus vaccine syringe 9| IPV vaccine administration, Module 4 | October 2020

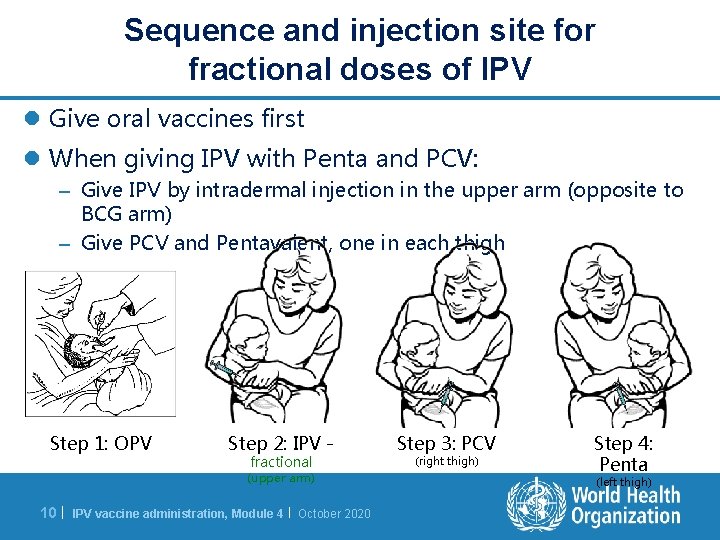
Sequence and injection site for fractional doses of IPV l Give oral vaccines first l When giving IPV with Penta and PCV: – Give IPV by intradermal injection in the upper arm (opposite to BCG arm) – Give PCV and Pentavalent, one in each thigh Step 1: OPV Step 2: IPV fractional (upper arm) 10 | IPV vaccine administration, Module 4 | October 2020 Step 3: PCV (right thigh) Step 4: Penta (left thigh)

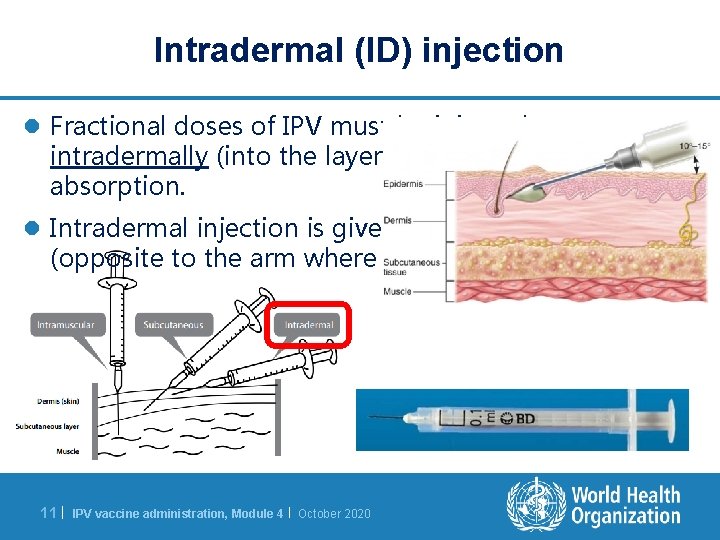
Intradermal (ID) injection l Fractional doses of IPV must be injected intradermally (into the layers of the skin) for slow absorption. l Intradermal injection is given in the upper arm (opposite to the arm where BCG was given). 11 | IPV vaccine administration, Module 4 | October 2020

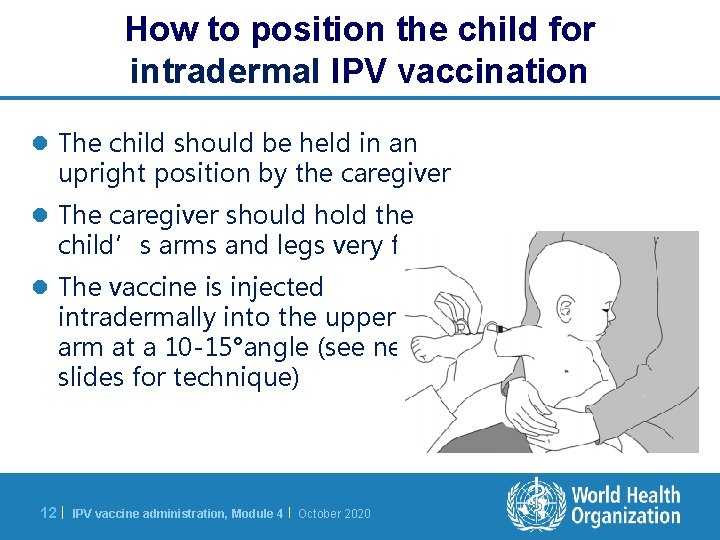
How to position the child for intradermal IPV vaccination l The child should be held in an upright position by the caregiver l The caregiver should hold the child’s arms and legs very firmly l The vaccine is injected intradermally into the upper arm at a 10 -15°angle (see next slides for technique) 12 | IPV vaccine administration, Module 4 | October 2020

How to administer intradermal IPV • Injection technique is similar to BCG. • To measure and inject the very small dose accurately, special 0. 1 m. L syringes should be used Technique: 1. Wash your hands well for 15 seconds 2. Hold the syringe barrel with fingers and thumb on the sides of the barrel with the bevel (hole) of the needle facing upwards 3. Lay the syringe and needle almost flat along the skin 4. Insert the tip of the needle under the surface of the skin, just past the bevel 5. Keep the needle close to the skin at the same angle as you inserted it 13 | IPV vaccine administration, Module 4 | October 2020

How to administer intradermal IPV 6. Place thumb on lower end of syringe near needle to hold position (but do not touch the needle!) 7. Hold the plunger end of the syringe between index and middle fingers. Press the plunger slowly with the thumb. If you feel no resistance to the plunger, you are not in the right place and should reposition 8. A pale flat-topped swelling (“bleb”) with small pits like an orange peel should appear on the skin 9. Remove needle slowly at same angle as it went in 10. Do not rub or massage the area 11. Discard the needle and syringe into safety box 14 | IPV vaccine administration, Module 4 | October 2020

How to administer intradermal IPV l When an intradermal injection is given correctly, the syringe plunger is hard to push. If the plunger goes in too easily, the injection may be too deep. l If this occurs, stop injecting immediately, correct the position of the needle, and give the remainder of the dose, but no more. l If the whole dose has already gone in, count the infant as having received a dose of vaccine, even though it was given subcutaneously rather than intradermally. l 15 Do not repeat the dose. | IPV vaccine administration, Module 4 | October 2020

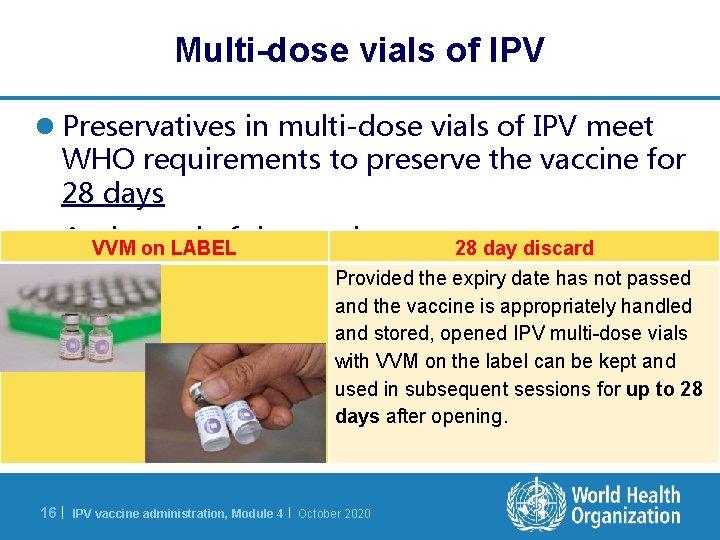
Multi-dose vials of IPV l Preservatives in multi-dose vials of IPV meet WHO requirements to preserve the vaccine for 28 days l At. VVM theonend of the session: LABEL 28 day discard Provided the expiry date has not passed and the vaccine is appropriately handled and stored, opened IPV multi-dose vials with VVM on the label can be kept and used in subsequent sessions for up to 28 days after opening. 16 | IPV vaccine administration, Module 4 | October 2020

Factors associated with vaccine wastage l Unavoidable – Requirement to discard opened multi-dose vials 28 days after opening l Avoidable – Poor stock management • Over-supply • Vaccine reaches expiry before use (recall the EEFO principle) • Lost, broken, stolen vials – Cold chain failure • Loss of potency (high temperatures) • Inactivated vaccine (freezing) – Poor vaccination technique • Administration of more than recommended dose (0. 1 m. L for fractional doses) for each injection 17 | IPV vaccine administration, Module 4 | October 2020

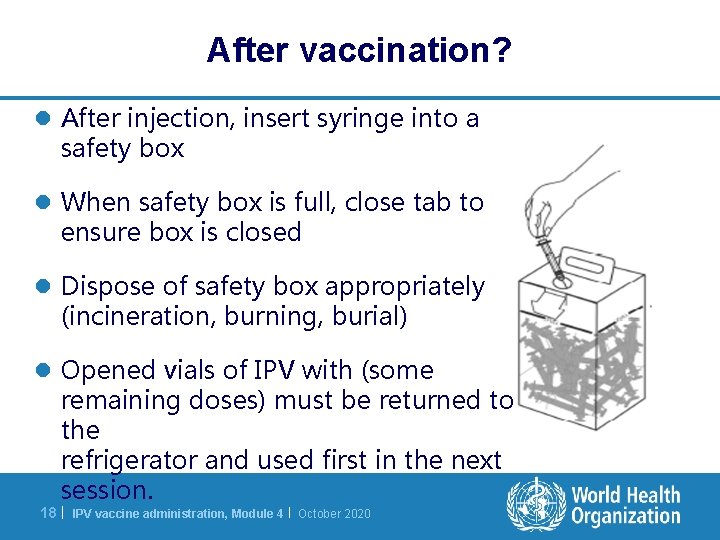
After vaccination? l After injection, insert syringe into a safety box l When safety box is full, close tab to ensure box is closed l Dispose of safety box appropriately (incineration, burning, burial) l Opened vials of IPV with (some remaining doses) must be returned to the refrigerator and used first in the next session. 18 | IPV vaccine administration, Module 4 | October 2020

What should you do in this scenario? What are some ways to reduce pain when giving an injection? 19 | IPV vaccine administration, Module 4 | October 2020

What should you do in this scenario? The child is 14 weeks old. You give him/her OPV, Rota, IPV, PCV and pentavalent vaccines. In which order should you give the vaccines? 20 | IPV vaccine administration, Module 4 | October 2020

Key messages l Check and interpret VVM and expiration date on the vaccine vial before giving the vaccine l IPV fractional dose is prepared and administered similarly to BCG (and other intradermal injections) – Prepare and dispose of IPV as you do other injectable vaccines l Have the caregiver comfortably hold the child upright while inserting the needle intradermally into the upper arm at a 10 -15⁰ angle l Give OPV first, then the intradermal injection (IPV), and then administer other injectable vaccines: IPV in the upper arm, and PCV and Pentavalent (one in each thigh) 21 | IPV vaccine administration, Module 4 | October 2020

End of module Thank you for your attention! 22 | IPV vaccine administration, Module 4 | October 2020
 Lab diagnosis of poliovirus
Lab diagnosis of poliovirus Poliovirus hominis
Poliovirus hominis Poliovirus structure
Poliovirus structure Formuö
Formuö Typiska novell drag
Typiska novell drag Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Ekologiskt fotavtryck
Ekologiskt fotavtryck Shingelfrisyren
Shingelfrisyren En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Personalliggare bygg undantag
Personalliggare bygg undantag Tidbok yrkesförare
Tidbok yrkesförare Sura för anatom
Sura för anatom Densitet vatten
Densitet vatten Datorkunskap för nybörjare
Datorkunskap för nybörjare Boverket ka
Boverket ka Debattinlägg mall
Debattinlägg mall Magnetsjukhus
Magnetsjukhus Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Påbyggnader för flakfordon
Påbyggnader för flakfordon Kraft per area
Kraft per area Svenskt ramverk för digital samverkan
Svenskt ramverk för digital samverkan Urban torhamn
Urban torhamn Presentera för publik crossboss
Presentera för publik crossboss











































