Training for Inactivated Poliovirus Vaccine IPV introduction Module















- Slides: 18

Training for Inactivated Poliovirus Vaccine (IPV) introduction Module 6 Inactivated poliovirus vaccine AEFI monitoring

Learning objectives l At the end of the module, the participants will be able to: – Identify adverse events following immunization (AEFIs) – Explain how to report AEFIs l Duration – 30 minutes 2| Inactivated poliovirus vaccine AEFI monitoring, Module 6 I 23 November 2020

Key issues 1 2 3 What is an AEFI? How likely is an AEFI after IPV? Which AEFIs do I report? 4 How do I report an AEFI? 3| Inactivated poliovirus vaccine AEFI monitoring, Module 6 I 23 November 2020

Adverse Event Following Immunization (AEFI) l What is an AEFI? – – A medical incident Takes place after an immunization Causes concern Is believed to be caused by immunization l How are AEFIs categorized? 4| Type Severity - Vaccine reaction - Programme error - Coincidental - Injection reaction - Unknown - Minor Severe • Serious • Non-serious … these are defined soon Inactivated poliovirus vaccine AEFI monitoring, Module 6 I 23 November 2020

Programme errors l Errors in vaccine preparation, handling, storage, or administration l Preventable l Often constitute the greatest proportion of AEFIs l Identification and correction are of great importance l Examples: – Non-sterile injection – Injection at incorrect site – Administration of frozen vaccine 5| Inactivated poliovirus vaccine AEFI monitoring, Module 6 I 23 November 2020

Coincidental events l Occur after vaccination, but not caused by the vaccine or its administration l Occur during infancy when illnesses are common and congenital or early neurological conditions become apparent l Onset temporally associated with vaccination, and inevitable when vaccinating these age groups l Applying normal incidence of disease and death in these age groups along with coverage allows estimation of expected numbers of coincidental events after immunization | Inactivated poliovirus vaccine AEFI monitoring, Module 6 6 I 23 November 2020

Injection reactions l Immunization anxiety-related reactions in anticipation to and as a result of an injection of any kind l Not related to the vaccine but to fear of the injection l You may encounter 4 types of injection reactions : – Fainting – Hyperventilation – Vomiting – Convulsions 7| Inactivated poliovirus vaccine AEFI monitoring, Module 6 I 23 November 2020

Vaccine reactions can be classified into two levels of severity l Minor reactions – Usually occur within few hours – Resolve quickly – Pose little danger – Local: pain, swelling, redness at injection site – Systemic: fever, malaise, muscle pain, headache, or loss of appetite 8| l Severe reactions- require timely and appropriate management – Usually do not result in longterm problems – Can be disabling – Are rarely life threatening – Include seizures and allergic reactions caused by body’s reaction to a particular component in a vaccine Inactivated poliovirus vaccine AEFI monitoring, Module 6 I 23 November 2020

Some severe events can be SERIOUS l A serious AEFI- Any untoward medical occurrence that at any dose: – Results in death – Is life threatening – Requires in-patient hospitalization or prolongation of existing hospitalization – Results in persistent or significant disability or incapacity 9| Inactivated poliovirus vaccine AEFI monitoring, Module 6 I 23 November 2020

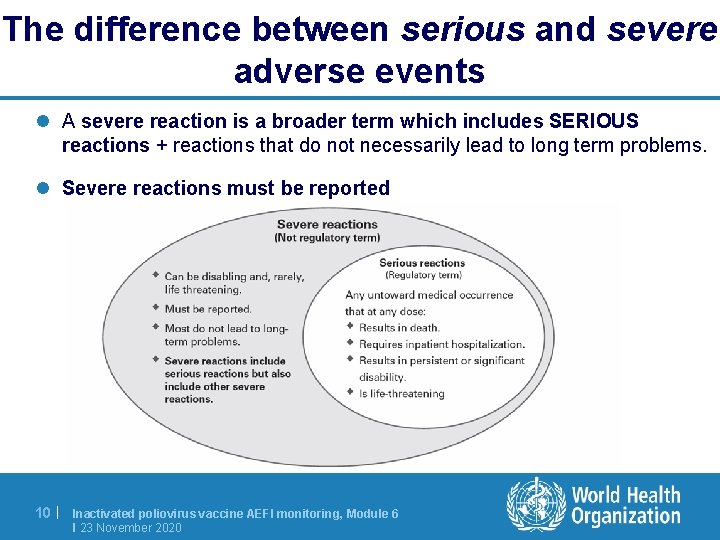
The difference between serious and severe adverse events l A severe reaction is a broader term which includes SERIOUS reactions + reactions that do not necessarily lead to long term problems. l Severe reactions must be reported 10 | Inactivated poliovirus vaccine AEFI monitoring, Module 6 I 23 November 2020

How likely is an AEFI after IPV? l IPV is a very safe vaccine l Not associated with serious systemic AEFIs l Inactivated vaccine – therefore, no risk of vaccine-associate polio l Can safely be administered with other recommended childhood vaccines, including OPV and other injectable vaccines 11 | Inactivated poliovirus vaccine AEFI monitoring, Module 6 I 23 November 2020

Which AEFIs should be reported? l Any severe events or AEFI that is of concern to the parents or health care worker 12 | Inactivated poliovirus vaccine AEFI monitoring, Module 6 I 23 November 2020

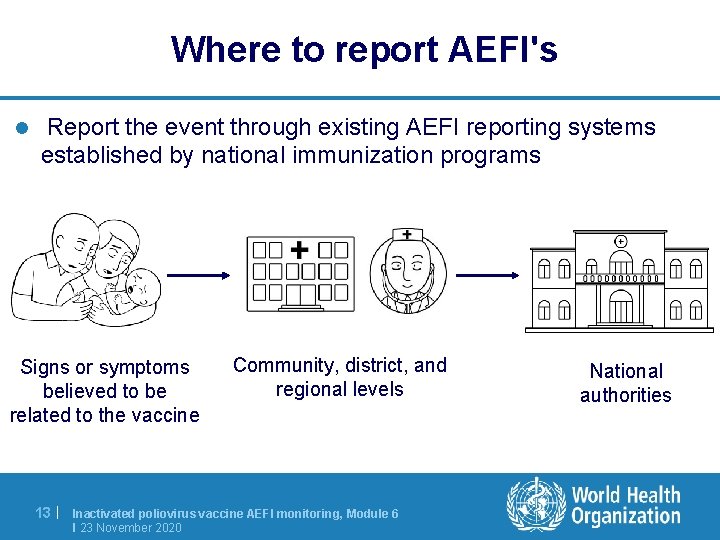
Where to report AEFI's l Report the event through existing AEFI reporting systems established by national immunization programs Signs or symptoms believed to be related to the vaccine 13 | Community, district, and regional levels Inactivated poliovirus vaccine AEFI monitoring, Module 6 I 23 November 2020 National authorities

Conducting an AEFI investigation l Some AEFI reports will need further investigation by the immunization programme managers l Components of AEFI investigation: – Identify specifications of implicated vaccine – Examine operational aspects of the programme which may have led to immunization errors – Search for other potential AEFI cases/clustering – Compare background risk to reported rate of AEFI – Confirm (or propose) the diagnosis and determine outcome – In collaboration with experts, determine if AEFI was vaccinerelated 14 | Inactivated poliovirus vaccine AEFI monitoring, Module 6 I 23 November 2020

What information should an AEFI report contain? l Client information l Details about the vaccine – – – Type Date Manufacturer Lot/expiration date Site/route of immunization, etc. l Description of adverse event(s) and any associated events l Medical and treatment history l Reporter details 15 | Inactivated poliovirus vaccine AEFI monitoring, Module 6 I 23 November 2020

Communication with caretakers in case of an AEFI l Reassure the caretaker l Admit uncertainty l Convey that the AEFI will be reported and investigated fully l Keep the community informed with follow-up information 16 | Inactivated poliovirus vaccine AEFI monitoring, Module 6 I 23 November 2020

Key messages l AEFIs can be minor or severe (which include serious events) l AEFIs should be reported through existing AEFI reporting systems/forms l The safety profile of IPV is excellent l Most infants who get IPV do not experience any side effects l In case of an AEFI – – 17 | Reassure the caretaker Admit uncertainty Investigate fully Keep the community informed Inactivated poliovirus vaccine AEFI monitoring, Module 6 I 23 November 2020

End of module Thank you for your attention! 18 | Inactivated poliovirus vaccine AEFI monitoring, Module 6 I 23 November 2020