Training for Inactivated Poliovirus Vaccine IPV introduction Module













- Slides: 17

Training for Inactivated Poliovirus Vaccine (IPV) introduction Module 6 Monitoring Events Supposedly Attributable to Vaccination or Immunization (ESAVIs)

Learning objectives l At the end of the module, the participants will be able to: – Identify Events Supposedly Attributable to Vaccination or Immunization (ESAVI) – Explain how to report ESAVIs l Duration – 30 minutes 2| Monitoring Events Supposedly Attributable to Vaccination or Immunization (ESAVIs) I Module 6 I February 2015

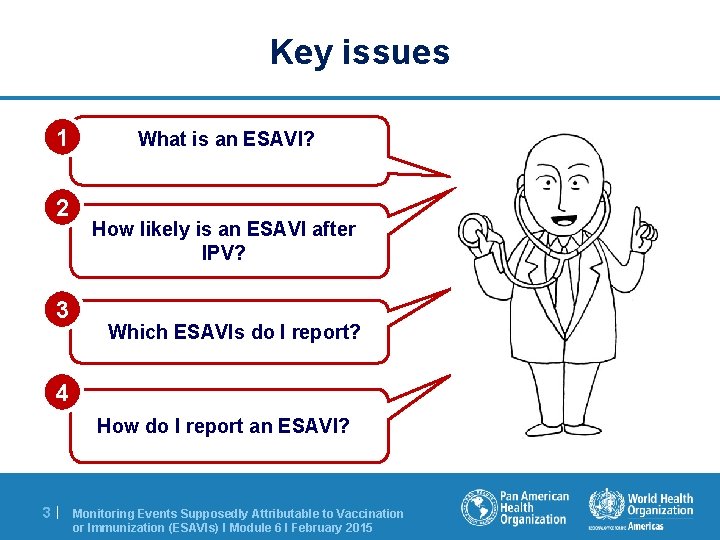
Key issues 1 2 3 What is an ESAVI? How likely is an ESAVI after IPV? Which ESAVIs do I report? 4 How do I report an ESAVI? 3| Monitoring Events Supposedly Attributable to Vaccination or Immunization (ESAVIs) I Module 6 I February 2015

Events Supposedly Attributable to Vaccination (ESAVI) l What is an ESAVI? – It is a clinical symptom that occurs after the administration of a vaccine, that may or may not be related, but may lead to concern if it is perceived to be attributable to vaccination. – While an ESAVI denotes a temporal assosiation, it does not necessarily imply a cause-effect relationship. The causality between the event and the vaccination is determined by the investigation. 4| Monitoring Events Supposedly Attributable to Vaccination or Immunization (ESAVIs) I Module 6 I February 2015

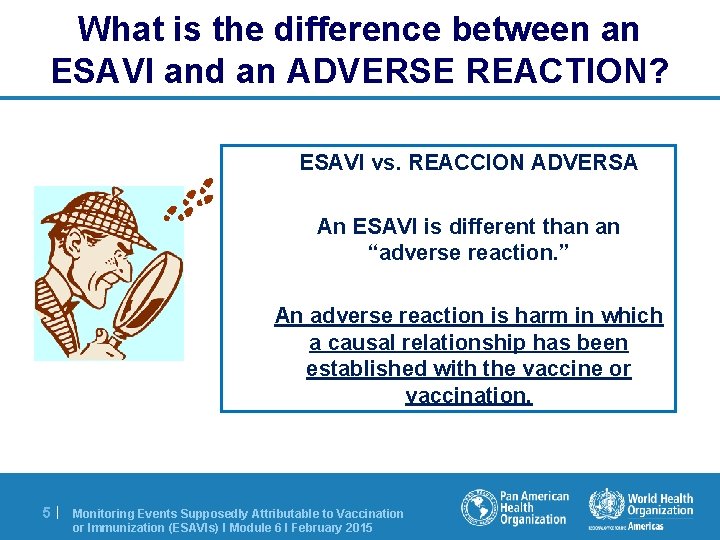
What is the difference between an ESAVI and an ADVERSE REACTION? ESAVI vs. REACCION ADVERSA An ESAVI is different than an “adverse reaction. ” An adverse reaction is harm in which a causal relationship has been established with the vaccine or vaccination. 5| Monitoring Events Supposedly Attributable to Vaccination or Immunization (ESAVIs) I Module 6 I February 2015

How do you classify ESAVIs? By type - Coincidental event (not related to the vaccines, but due to another cause) - Vaccine-related reaction • Programmatic errors • Vaccine product related reaction - Anxiety-related reaction - Inconclusive events 6| Monitoring Events Supposedly Attributable to Vaccination or Immunization (ESAVIs) I Module 6 I February 2015 By severity - Mild (most - common) Moderate Severe (least common)

Difference between “severe” and “serious” l A “severe” ESAVI – Describes the intensity of a specific event (ie: mild, moderate, or severe). – Can be a relatively minor medical importance. 7| l A “serious” ESAVI – – Could result in death Potentially be life-threatening Require hospitalization Result in persistent or significant disability – Require intervention to prevent permanent impairment or damage. Monitoring Events Supposedly Attributable to Vaccination or Immunization (ESAVIs) I Module 6 I February 2015

Coincidental events l Occur after vaccination, but not caused by the vaccine or its administration l The administration of most vaccines coincides with the period of greatest vulnerability of children when illnesses are common and congenital or early neurological conditions become apparent 8| Monitoring Events Supposedly Attributable to Vaccination or Immunization (ESAVIs) I Module 6 I February 2015

Programmatic errors l Errors in the preparation, handling, storage or administration of the vaccine. l Not fulfilling the standards set by the National Program. l They are preventable. l They often represent most ESAVIs. l Errors in training l It is critical to recognize and correct these errors: – Examples: • Unsterile injection. • Injection in the wrong place. • Administration of vaccine that has passed its expiration date. 9| Monitoring Events Supposedly Attributable to Vaccination or Immunization (ESAVIs) I Module 6 I February 2015

How likely is an ESAVI after IPV? l IPV is a very safe vaccine and well tolerated. l Adverse reactions are extremely rare. l It is an inactivated vaccine – therefore, no risk of vaccine-associate polio l Contraindications of polio are: • Documented allergy to streptomycin, neomycin, or polymyxin B • History of an allergic reaction following a previous IPV injection 10 | Monitoring Events Supposedly Attributable to Vaccination or Immunization (ESAVIs) I Module 6 I February 2015

Which ESAVIs should be reported? • Any ESAVI that is concern to parents or health-care workers should be reported. • In particular, health care workers should report: • • “Serious” ESAVIs Signals and events associated with a newly introduced vaccine ESAVIs that may have been caused by a programmatic error Significant events of unexplained causes occurring within 30 days after vaccination • Unexpected events in which the relation to the vaccination is unclear • Clustered events The role of the notifier is to notify and NOT assess causality. 11 | Monitoring Events Supposedly Attributable to Vaccination or Immunization (ESAVIs) I Module 6 I February 2015

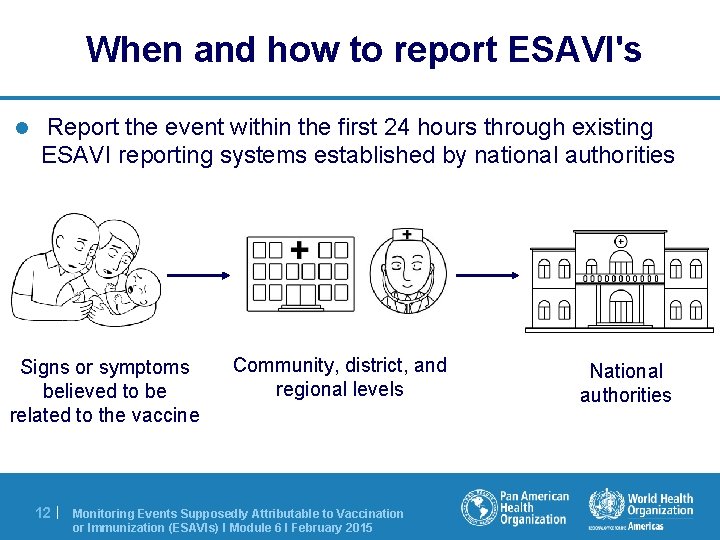
When and how to report ESAVI's l Report the event within the first 24 hours through existing ESAVI reporting systems established by national authorities Signs or symptoms believed to be related to the vaccine 12 | Community, district, and regional levels Monitoring Events Supposedly Attributable to Vaccination or Immunization (ESAVIs) I Module 6 I February 2015 National authorities

Conducting an ESAVI investigation l ESAVIs should be investigated within the first 48 hours of notification of the case l Some ESAVIs require further investigation by national authorities and committees ESAVI. l Components of ESAVI investigation: – Identify specifications of implicated vaccine – Examine operational aspects of the program which may have led to immunization errors – Determine if case is isolated or if there are related cases – Verify if there are cases in non-vaccinated people – Compare background risk to reported rate of ESAVI – In collaboration with experts, determine if ESAVI was vaccine-related 13 | Monitoring Events Supposedly Attributable to Vaccination or Immunization (ESAVIs) I Module 6 I February 2015

What information should an ESAVI report contain? l Client information l Details about the vaccine – – – Type Date Manufacturer Lot/expiration date Site/route of immunization, etc. l Description of adverse event(s) l Medical and treatment history l Reporter details 14 | Monitoring Events Supposedly Attributable to Vaccination or Immunization (ESAVIs) I Module 6 I February 2015

Communication with caretakers in case of an ESAVI l Reassure the caretakers and keep them informed them on the progress of the investigation. l Convey that the ESAVI will be reported and investigated fully. l Keep the community and health workers informed on the investigation. 15 | Monitoring Events Supposedly Attributable to Vaccination or Immunization (ESAVIs) I Module 6 I February 2015

Key messages l ESAVIs are generally mild l ESAVIs in rare situations can be serious l ESAVIs should be reported through existing ESAVI reporting systems/forms l IPV is a very safe vaccine l Most infants who get IPV do not experience any side effects. Serious reactions are extremely rare. l In case of an ESAVI – – 16 | Ensure immediate attention to the child Reassure the caretaker Investigate fully Keep the community informed Monitoring Events Supposedly Attributable to Vaccination or Immunization (ESAVIs) I Module 6 I February 2015

End of module Thank you for your attention! 17 | Monitoring Events Supposedly Attributable to Vaccination or Immunization (ESAVIs) I Module 6 I February 2015