Todays Menu Why study nuclear physics Why nuclear








- Slides: 38

Today’s Menu • • Why study nuclear physics Why nuclear physics is difficult Course synopsis. Notation & Units Tony Weidberg Nuclear Physics Lectures 1

Why Study Nuclear Physics? • Understand origin of different nuclei – Big bang: H, He and Li – Stars: elements up to Fe – Supernova: heavy elements • We are all made of stardust • Need to know nuclear cross sections experimental nuclear astrophysics is a hot topic. Tony Weidberg Nuclear Physics Lectures 2

Practical Applications • Nuclear fission for energy generation. – No greenhouse gasses – Safety and storage of radioactive material. • Nuclear fusion – No safety issue (not a bomb) – Less radioactive material but still some. • Nuclear transmutation of radioactive waste with neutrons. – Turn long lived isotopes stable or short lived. • Every physicist should have an informed opinion on these important issues! Tony Weidberg Nuclear Physics Lectures 3

Medical Applications • Radiotherapy for cancer – Kill cancer cells. – Used for 100 years but can be improved by better delivery and dosimetery – Heavy ion beams can give more localised energy deposition. • Medical Imaging – – MRI (Nuclear magnetic resonance) X-rays (better detectors lower doses) PET Many others…see Medical & Environmental short option. Tony Weidberg Nuclear Physics Lectures 4

Other Applications • Radioactive Dating – C 14/C 12 gives ages for dead plants/animals/people. – Rb/Sr gives age of earth as 4. 5 Gyr. • Element analysis – Foresenic (eg date As in hair). – Biology (eg elements in blood cells) – Archaeology (eg provenance via isotope ratios). Tony Weidberg Nuclear Physics Lectures 5

Why is Nuclear Physics Hard? • QCD theory of strong interactions just solve the equations … • At short distance/large Q coupling constant small perturbation theory ok but long distance/small Q, q large Tony Weidberg Nuclear Physics Lectures 6

Nuclear Physics Models • Progress with understanding nuclear physics from QCD=0 use simple, approximate, phenomenological models. • Liquid Drop Model: phenomenology + QM + EM. • Shell Model: look at quantum states of individual nucleons understand spin/parity magnetic moments and deviations from SEMF for binding energy. Tony Weidberg Nuclear Physics Lectures 7

Course Synopsis - 1 • Liquid Drop Model and SEMF. • Applications of SEMF – Valley of stability. – abg decays. – Fission & fusion. • Shell Model – Magic numbers, spin/parity, magnetic moments. Tony Weidberg Nuclear Physics Lectures 8

Course Synopsis - 2 • Cross Sections – Experimental definition – FGR theory – Rutherford scattering – Breit-Wigner resonances • Theory of abg decays. • Particle interactions in matter – Simple detectors for nuclear/particle physics. Tony Weidberg Nuclear Physics Lectures 9

What is the use of lectures • Definition of a lecture: a process whereby notes are transferred from the pages of a lecturer to the pages of the student without passing through the head of either. • Conclusion: to make lectures useful YOU have to participate, ask questions ! If you don’t understand something the chances are >50% of the audience doesn’t as well, so don’t be shy ! Tony Weidberg Nuclear Physics Lectures 10

Corrections • To err is human … and this is a new course lots of mistakes. • Please tell me about any mistakes you find in the notes (I will donate a bottle of wine to the person who finds the most mistakes!). Tony Weidberg Nuclear Physics Lectures 11

The Minister of Science • This is a true story honest. • Once upon a time the government science minister visited the Rutherford Lab (UK national lab) and after a days visit of the lab was discussing his visit with the lab director and he said … • I hope that you all have a slightly better grasp of the subject by the end! Tony Weidberg Nuclear Physics Lectures 12

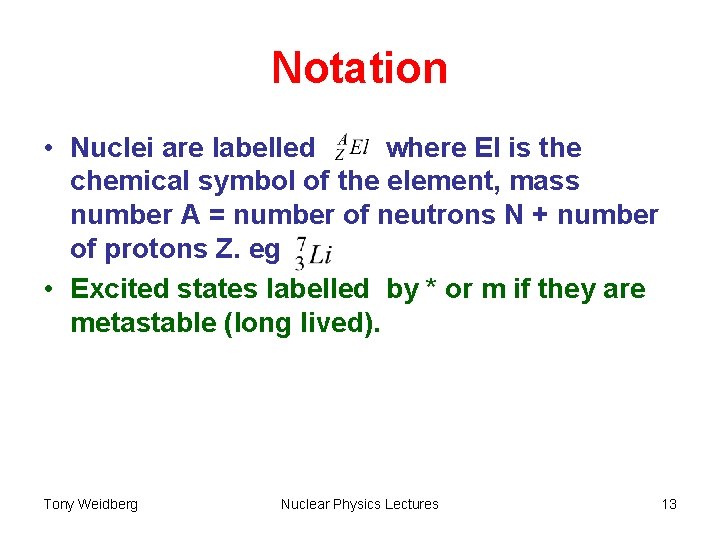
Notation • Nuclei are labelled where El is the chemical symbol of the element, mass number A = number of neutrons N + number of protons Z. eg • Excited states labelled by * or m if they are metastable (long lived). Tony Weidberg Nuclear Physics Lectures 13

Units • SI units are fine for macroscopic objects like footballs but are very inconvenient for nuclei and particles use natural units. Energy: 1 e. V = energy gained by electron in being accelerated by 1 V. • – • Mass: Me. V/c 2 (or Ge. V/c 2) – – • 1 e. V/c 2 = e/c 2 kg. Or use AMU defined by mass of 12 C= 12 u Momentum: Me. V/c (or Ge. V/c) – • 1 e. V/c = e/c kg m s-1 Cross sections: (as big as a barn door) – • 1 e. V= e J. 1 barn =10 -28 m 2 Length: fermi 1 fm = 10 -15 m. Tony Weidberg Nuclear Physics Lectures 14

Nuclear Masses and Sizes • Masses and binding energies – Absolute values measured with mass spectrometers. – Relative values from reactions and decays. • Nuclear Sizes – Measured with scattering experiments (leave discussion until after we have looked at Rutherford scattering). – Isotope shifts Tony Weidberg Nuclear Physics Lectures 15

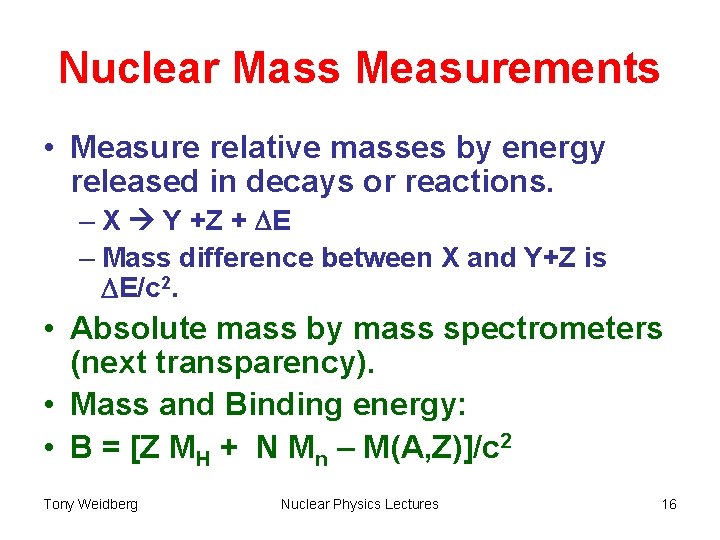
Nuclear Mass Measurements • Measure relative masses by energy released in decays or reactions. – X Y +Z + DE – Mass difference between X and Y+Z is DE/c 2. • Absolute mass by mass spectrometers (next transparency). • Mass and Binding energy: • B = [Z MH + N Mn – M(A, Z)]/c 2 Tony Weidberg Nuclear Physics Lectures 16

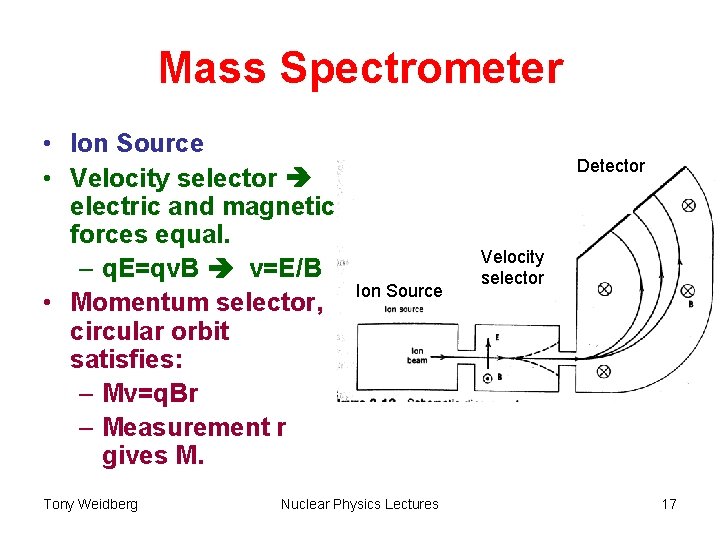
Mass Spectrometer • Ion Source • Velocity selector electric and magnetic forces equal. – q. E=qv. B v=E/B • Momentum selector, circular orbit satisfies: – Mv=q. Br – Measurement r gives M. Tony Weidberg Detector Ion Source Nuclear Physics Lectures Velocity selector 17

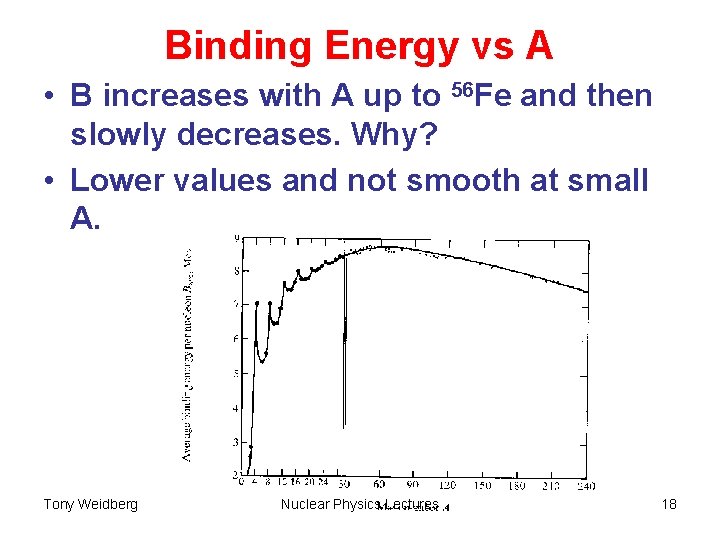
Binding Energy vs A • B increases with A up to 56 Fe and then slowly decreases. Why? • Lower values and not smooth at small A. Tony Weidberg Nuclear Physics Lectures 18

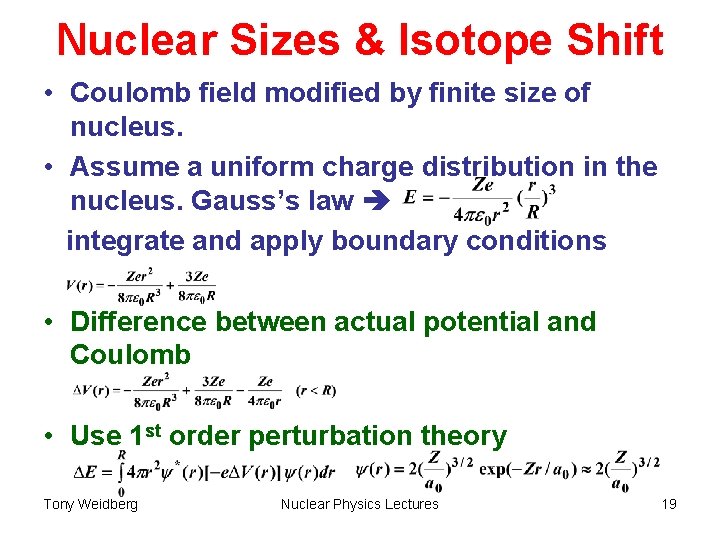
Nuclear Sizes & Isotope Shift • Coulomb field modified by finite size of nucleus. • Assume a uniform charge distribution in the nucleus. Gauss’s law integrate and apply boundary conditions • Difference between actual potential and Coulomb • Use 1 st order perturbation theory Tony Weidberg Nuclear Physics Lectures 19

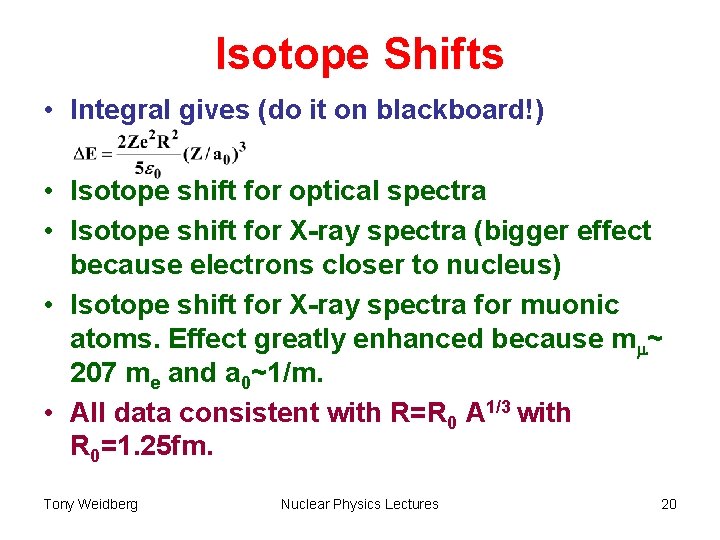
Isotope Shifts • Integral gives (do it on blackboard!) • Isotope shift for optical spectra • Isotope shift for X-ray spectra (bigger effect because electrons closer to nucleus) • Isotope shift for X-ray spectra for muonic atoms. Effect greatly enhanced because mm~ 207 me and a 0~1/m. • All data consistent with R=R 0 A 1/3 with R 0=1. 25 fm. Tony Weidberg Nuclear Physics Lectures 20

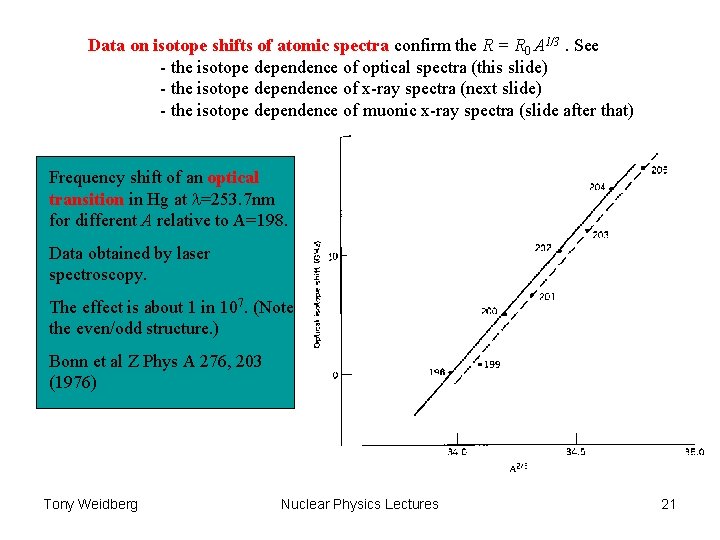
Data on isotope shifts of atomic spectra confirm the R = R 0 A 1/3. See - the isotope dependence of optical spectra (this slide) - the isotope dependence of x-ray spectra (next slide) - the isotope dependence of muonic x-ray spectra (slide after that) Frequency shift of an optical transition in Hg at =253. 7 nm for different A relative to A=198. Data obtained by laser spectroscopy. The effect is about 1 in 107. (Note the even/odd structure. ) Bonn et al Z Phys A 276, 203 (1976) Tony Weidberg Nuclear Physics Lectures 21

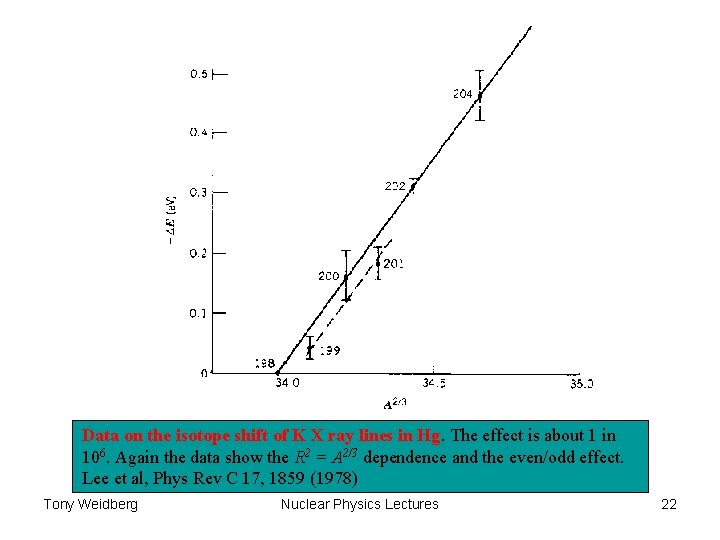
Data on the isotope shift of K X ray lines in Hg. The effect is about 1 in 106. Again the data show the R 2 = A 2/3 dependence and the even/odd effect. Lee et al, Phys Rev C 17, 1859 (1978) Tony Weidberg Nuclear Physics Lectures 22

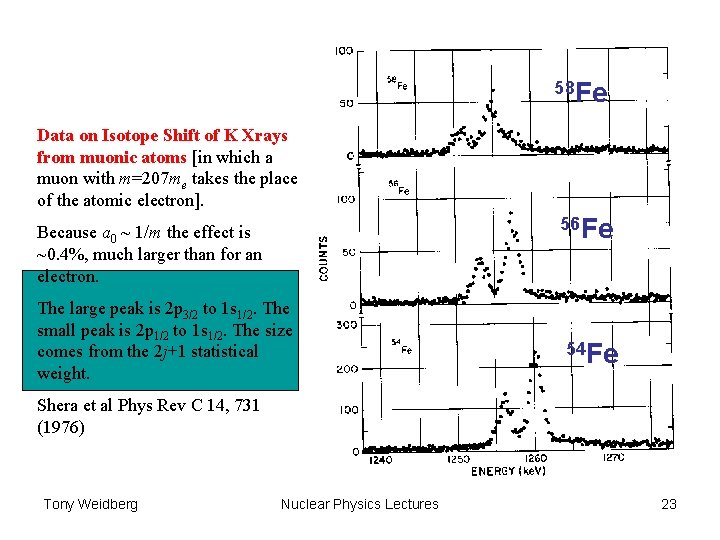
58 Fe Data on Isotope Shift of K Xrays from muonic atoms [in which a muon with m=207 me takes the place of the atomic electron]. 56 Fe Because a 0 ~ 1/m the effect is ~0. 4%, much larger than for an electron. The large peak is 2 p 3/2 to 1 s 1/2. The small peak is 2 p 1/2 to 1 s 1/2. The size comes from the 2 j+1 statistical weight. 54 Fe Shera et al Phys Rev C 14, 731 (1976) Tony Weidberg Nuclear Physics Lectures 23

SEMF • Aim: phenomenological understanding of nuclear binding energies as function of A & Z. • Nuclear density constant (see lecture 1). • Model effect of short range attraction due to strong interaction by liquid drop model. • Coulomb corrections. • Fermi gas model asymmetry term. • QM pairing term. • Compare with experiment: success & failure! Tony Weidberg Nuclear Physics Lectures 24

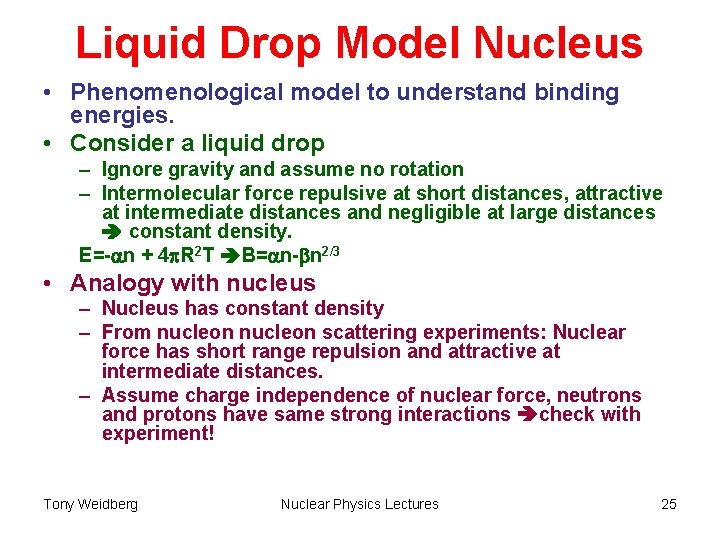
Liquid Drop Model Nucleus • Phenomenological model to understand binding energies. • Consider a liquid drop – Ignore gravity and assume no rotation – Intermolecular force repulsive at short distances, attractive at intermediate distances and negligible at large distances constant density. E=-an + 4 p. R 2 T B=an-bn 2/3 • Analogy with nucleus – Nucleus has constant density – From nucleon scattering experiments: Nuclear force has short range repulsion and attractive at intermediate distances. – Assume charge independence of nuclear force, neutrons and protons have same strong interactions check with experiment! Tony Weidberg Nuclear Physics Lectures 25

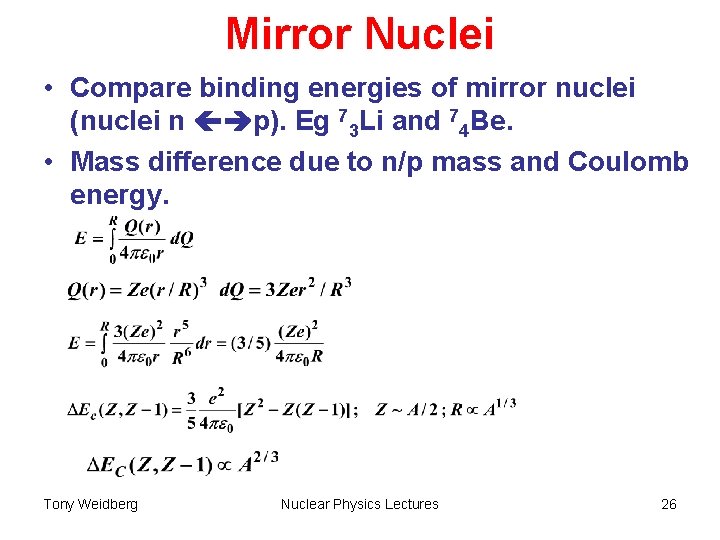
Mirror Nuclei • Compare binding energies of mirror nuclei (nuclei n p). Eg 73 Li and 74 Be. • Mass difference due to n/p mass and Coulomb energy. Tony Weidberg Nuclear Physics Lectures 26

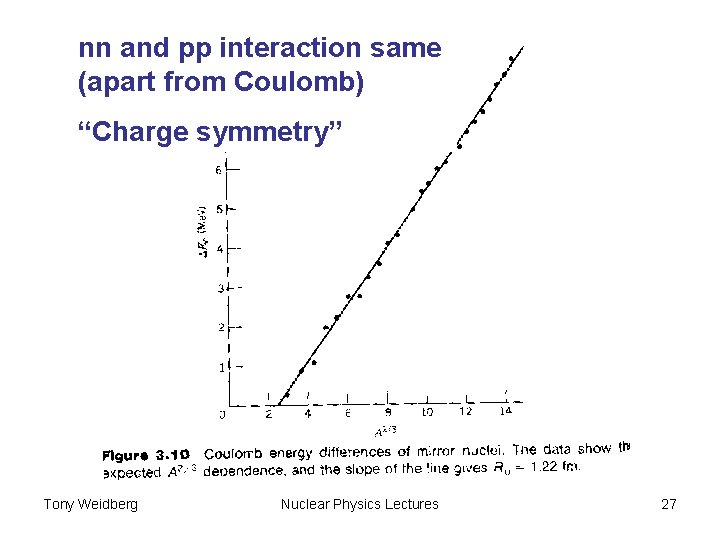
nn and pp interaction same (apart from Coulomb) “Charge symmetry” Tony Weidberg Nuclear Physics Lectures 27

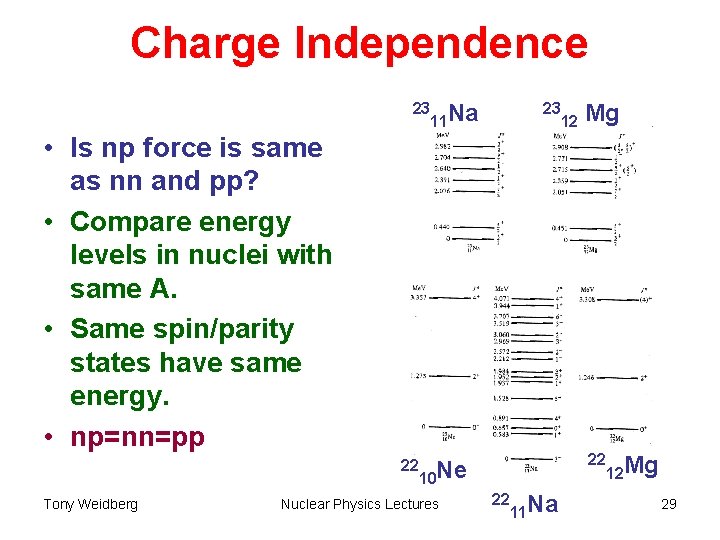
Charge Symmetry and Charge Independence • Mirror nuclei showed that strong interaction is the same for nn and pp. • What about np ? • Compare energy levels in “triplets” with same A, different number of n and p. e. g. • Same energy levels for the same spin states SI same for np as nn and pp. Tony Weidberg Nuclear Physics Lectures 28

Charge Independence 23 11 Na 23 • Is np force is same as nn and pp? • Compare energy levels in nuclei with same A. • Same spin/parity states have same energy. • np=nn=pp 22 Tony Weidberg Mg 22 10 Ne Nuclear Physics Lectures 12 22 11 Na 12 Mg 29

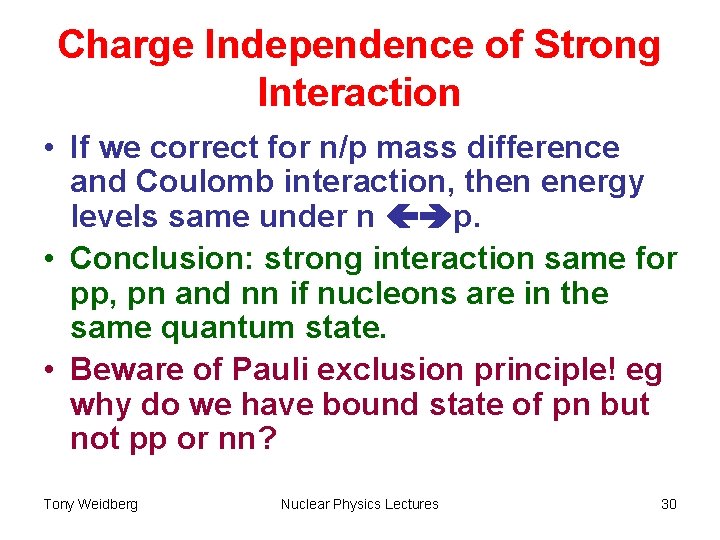
Charge Independence of Strong Interaction • If we correct for n/p mass difference and Coulomb interaction, then energy levels same under n p. • Conclusion: strong interaction same for pp, pn and nn if nucleons are in the same quantum state. • Beware of Pauli exclusion principle! eg why do we have bound state of pn but not pp or nn? Tony Weidberg Nuclear Physics Lectures 30

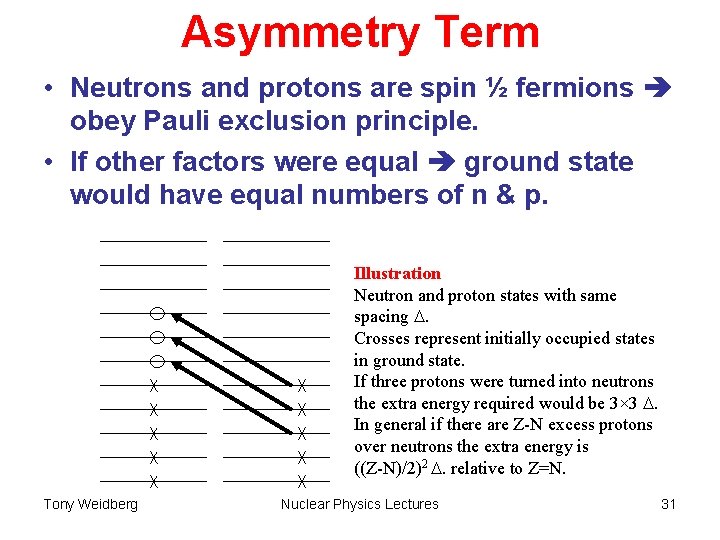
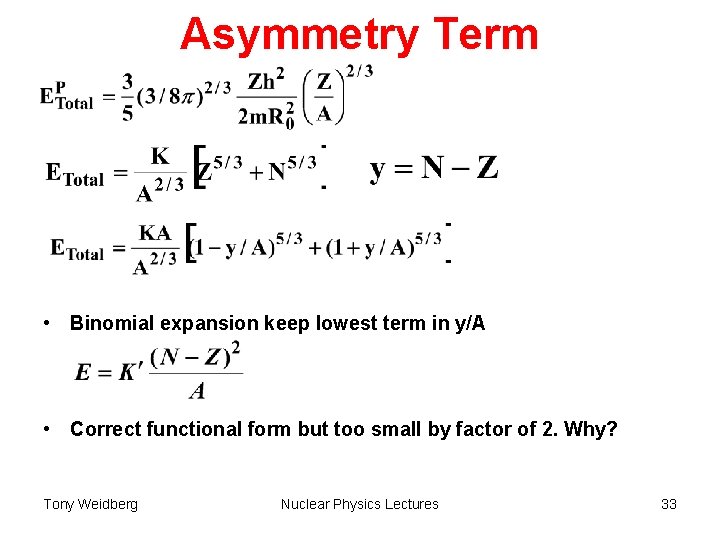
Asymmetry Term • Neutrons and protons are spin ½ fermions obey Pauli exclusion principle. • If other factors were equal ground state would have equal numbers of n & p. Illustration Neutron and proton states with same spacing . Crosses represent initially occupied states in ground state. If three protons were turned into neutrons the extra energy required would be 3× 3 . In general if there are Z-N excess protons over neutrons the extra energy is ((Z-N)/2)2 . relative to Z=N. Tony Weidberg Nuclear Physics Lectures 31

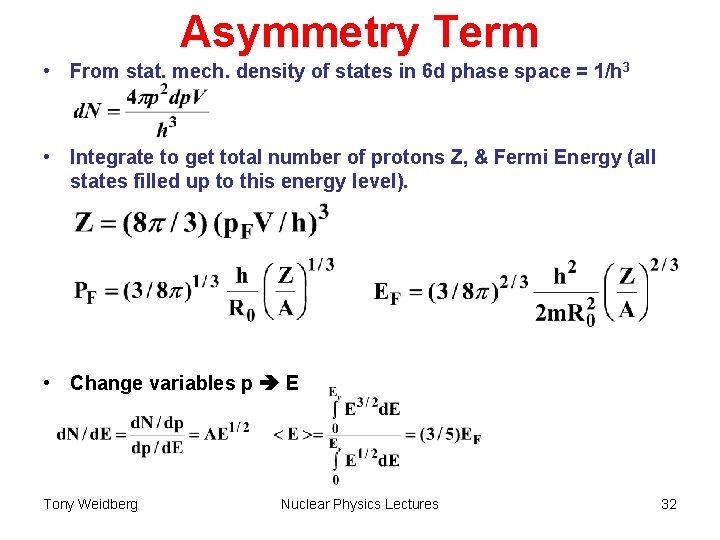
Asymmetry Term • From stat. mech. density of states in 6 d phase space = 1/h 3 • Integrate to get total number of protons Z, & Fermi Energy (all states filled up to this energy level). • Change variables p E Tony Weidberg Nuclear Physics Lectures 32

Asymmetry Term • Binomial expansion keep lowest term in y/A • Correct functional form but too small by factor of 2. Why? Tony Weidberg Nuclear Physics Lectures 33

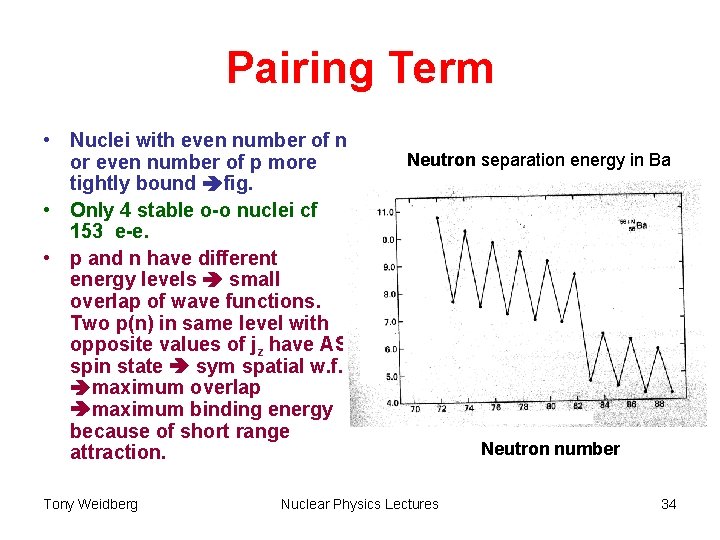
Pairing Term • Nuclei with even number of n or even number of p more tightly bound fig. • Only 4 stable o-o nuclei cf 153 e-e. • p and n have different energy levels small overlap of wave functions. Two p(n) in same level with opposite values of jz have AS spin state sym spatial w. f. maximum overlap maximum binding energy because of short range attraction. Tony Weidberg Neutron separation energy in Ba Nuclear Physics Lectures Neutron number 34

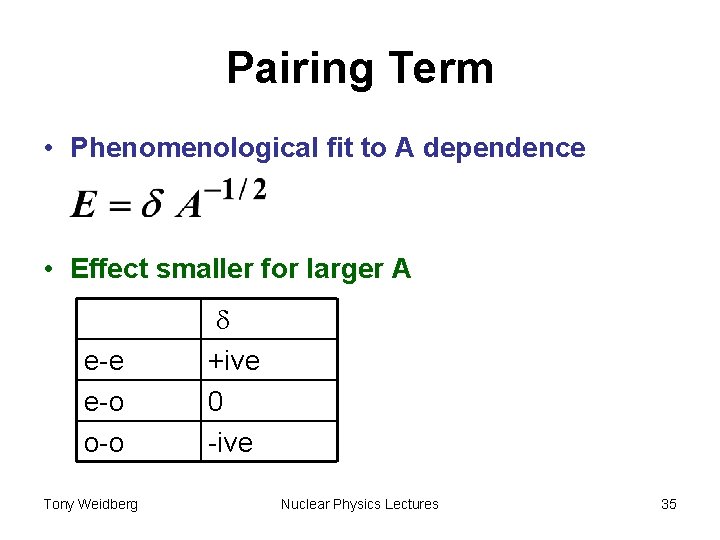
Pairing Term • Phenomenological fit to A dependence • Effect smaller for larger A e-e e-o o-o Tony Weidberg d +ive 0 -ive Nuclear Physics Lectures 35

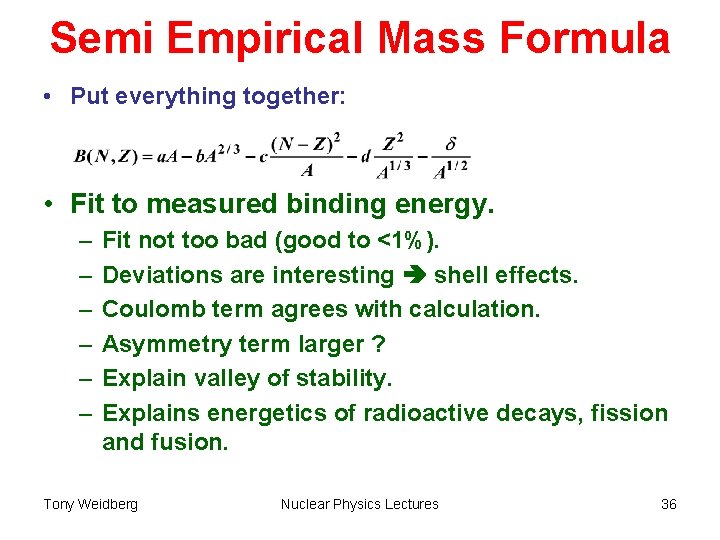
Semi Empirical Mass Formula • Put everything together: • Fit to measured binding energy. – – – Fit not too bad (good to <1%). Deviations are interesting shell effects. Coulomb term agrees with calculation. Asymmetry term larger ? Explain valley of stability. Explains energetics of radioactive decays, fission and fusion. Tony Weidberg Nuclear Physics Lectures 36

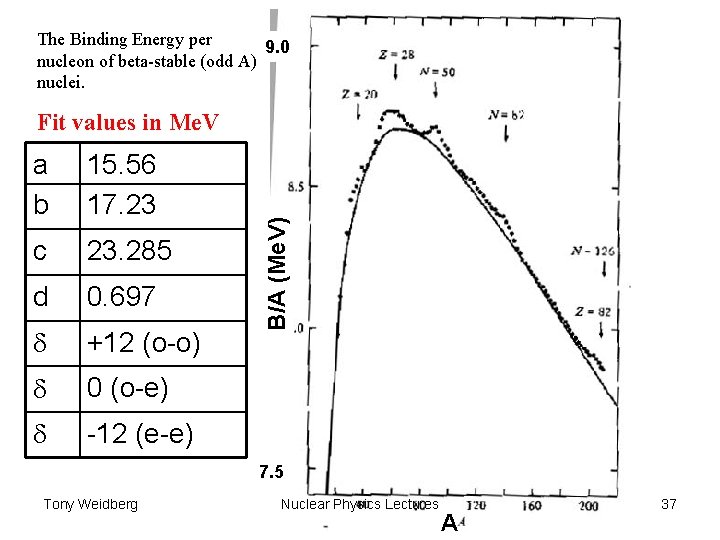
The Binding Energy per 9. 0 nucleon of beta-stable (odd A) nuclei. a b 15. 56 17. 23 c 23. 285 d 0. 697 d +12 (o-o) d 0 (o-e) d -12 (e-e) B/A (Me. V) Fit values in Me. V 7. 5 Tony Weidberg Nuclear Physics Lectures A 37

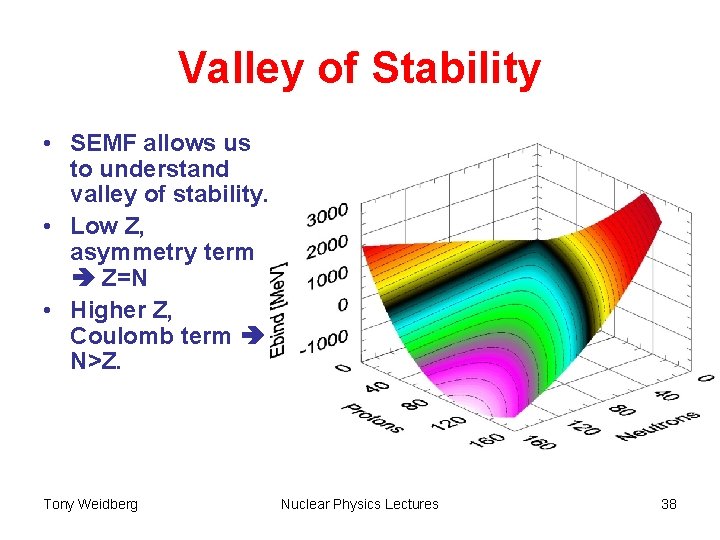
Valley of Stability • SEMF allows us to understand valley of stability. • Low Z, asymmetry term Z=N • Higher Z, Coulomb term N>Z. Tony Weidberg Nuclear Physics Lectures 38