Chapter 29 Nuclear Physics Nuclear Physics Sections 1














- Slides: 27

Chapter 29 Nuclear Physics

Nuclear Physics Sections 1– 4 General Physics

Milestones in Nuclear Physics n 1896 – the birth of nuclear physics n n 1911 Rutherford, Geiger and Marsden performed scattering experiments n n n Becquerel discovered radioactivity in uranium compounds Established the point mass nature of the nucleus Nuclear force was a new type of force 1919 Rutherford and coworkers first observed nuclear reactions n Naturally occurring alpha particles bombarded nitrogen nuclei to produce oxygen General Physics

History n n n Becquerel – discovered radioactivity (1896) (from radiare = emit rays) Curie – discovery of polonium and radium (1898) Rutherford – nuclear model n n Mosley – studied nucleus via X-ray spectra n n n n correlated (Z = charge of nucleus) with periodic table extra particles in nucleus: A = Z + ? Chadwick – discovered neutron (1932) Pauli – postulated neutral particle from -decay (1930) Fermi – theory or weak decay (1933) ‘neutrino’ Fission – Hahn, Strassmann, (&Meitner!) (1938) n n classified , , radiation, particle = 4 He nucleus (nuclear transmutation) used scattering to discover the nuclear model postulated ‘neutrons’ A=Z+N (1920); bound p+ e- state? first reactor (chain reaction), Fermi (1942) Bohr, Wheeler – liquid drop model Mayer, Jensen – shell model (1949) Hofstadter – electron scattering (1953 -) n n measured the charge density of various nuclei discovered structure in the proton (not point-like particle) General Physics

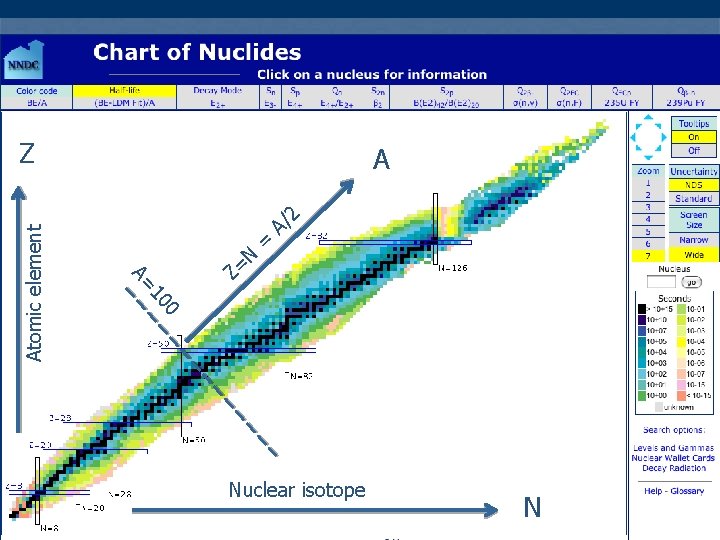
Some Properties of Nuclei n All nuclei are composed of protons and neutrons n n Atomic number, Z n n Number of protons in the nucleus Neutron number, N n n Exception is ordinary hydrogen just a proton Number of neutrons in the nucleus Mass number, A n n n Number of nucleons in the nucleus: A = Z + N Nucleon is a generic term used to refer to either a proton or a neutron in the nucleus The mass number is not the same as the mass General Physics with

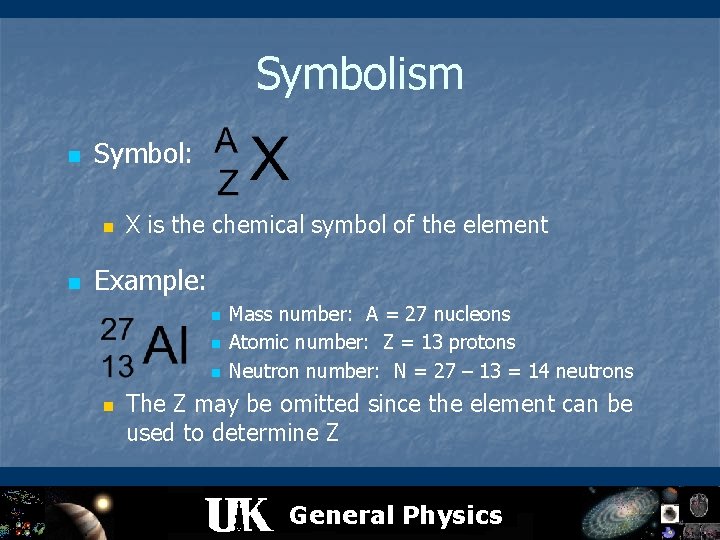
Symbolism n Symbol: n n X is the chemical symbol of the element Example: n n Mass number: A = 27 nucleons Atomic number: Z = 13 protons Neutron number: N = 27 – 13 = 14 neutrons The Z may be omitted since the element can be used to determine Z General Physics

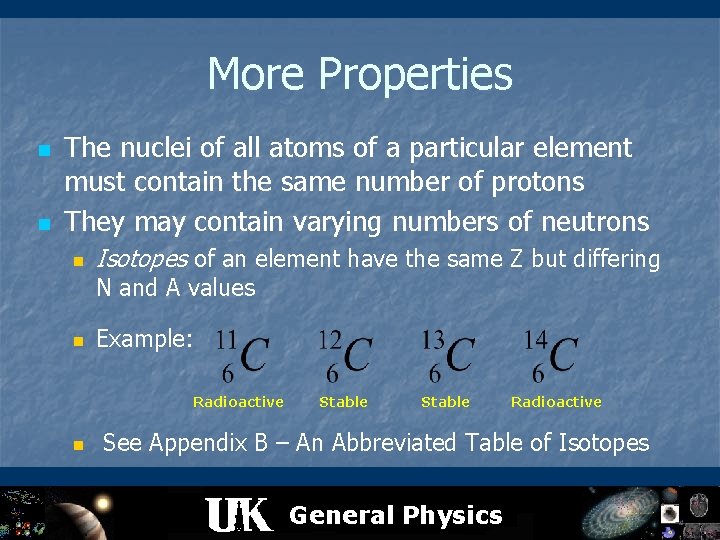
More Properties n n The nuclei of all atoms of a particular element must contain the same number of protons They may contain varying numbers of neutrons n Isotopes of an element have the same Z but differing N and A values n Example: Radioactive n Stable Radioactive See Appendix B – An Abbreviated Table of Isotopes General Physics

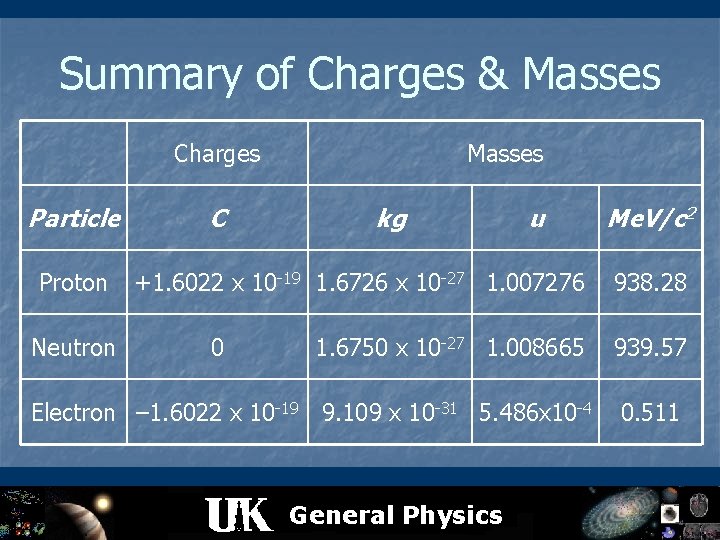
Charge and Mass n The proton has a single positive charge, +e n n e = 1. 60217733 x 10 -19 C The electron has a single negative charge, –e The neutron has no charge – difficult to detect unified mass unit: u n n n 1 u = 1. 660559 x 10 -27 kg Based on definition that mass of one atom of E = m c 2 1 u = 931. 494 Me. V/c 2 General Physics 12 C is exactly 12 u

Summary of Charges & Masses Charges Particle Proton Neutron C Masses kg u +1. 6022 x 10 -19 1. 6726 x 10 -27 1. 007276 0 Me. V/c 2 938. 28 1. 6750 x 10 -27 1. 008665 939. 57 Electron – 1. 6022 x 10 -19 9. 109 x 10 -31 5. 486 x 10 -4 0. 511 General Physics

Binding Energy n The total energy of the bound system (the nucleus) is less than the combined energy of the separated nucleons n This difference in energy is called the binding energy of the nucleus n It can be thought of as the amount of energy you need to add to the nucleus to break it apart into separated protons and neutrons General Physics

Binding Energy per Nucleon n n Except for light nuclei, the binding energy is ~ 8 Me. V per nucleon The curve peaks in the vicinity of A = 60 n n Nuclei with mass numbers greater than or less than 60 are not as strongly bound as those near the middle of the periodic table The curve is slowly varying at A > 40 General Physics

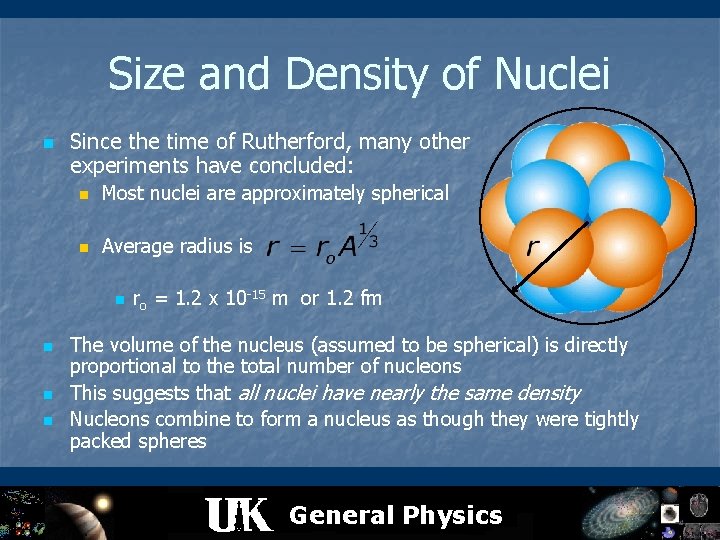
Size and Density of Nuclei n Since the time of Rutherford, many other experiments have concluded: n Most nuclei are approximately spherical n Average radius is n n ro = 1. 2 x 10 -15 m or 1. 2 fm The volume of the nucleus (assumed to be spherical) is directly proportional to the total number of nucleons This suggests that all nuclei have nearly the same density Nucleons combine to form a nucleus as though they were tightly packed spheres General Physics

A 2 N Z= = A/ 0 10 A= Atomic element Z Nuclear isotope General Physics N

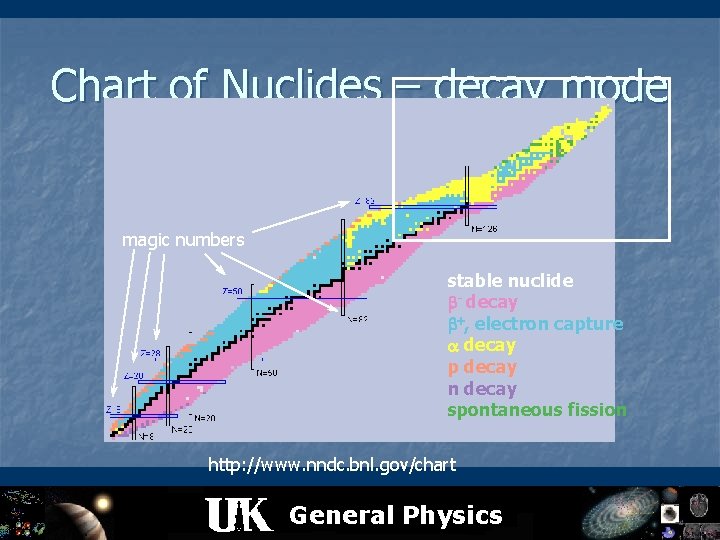
Nuclear decay modes: n n n n ISOTONES n S AR n OB n ++ decay - decay (isobar) + decay (isobar) electron capture (isobar) p decay (isotone) n decay (isotope) decay (isomers) electron conversion (EC) spontaneous fission (SF) double beta decay (2 ) neutrino-less double beta decay (0 ) beta-delayed n, p, decay IS n ISOTOPES Z General Physics ISOMERS N

Chart of Nuclides – decay mode magic numbers stable nuclide - decay , electron capture decay p decay n decay spontaneous fission http: //www. nndc. bnl. gov/chart General Physics

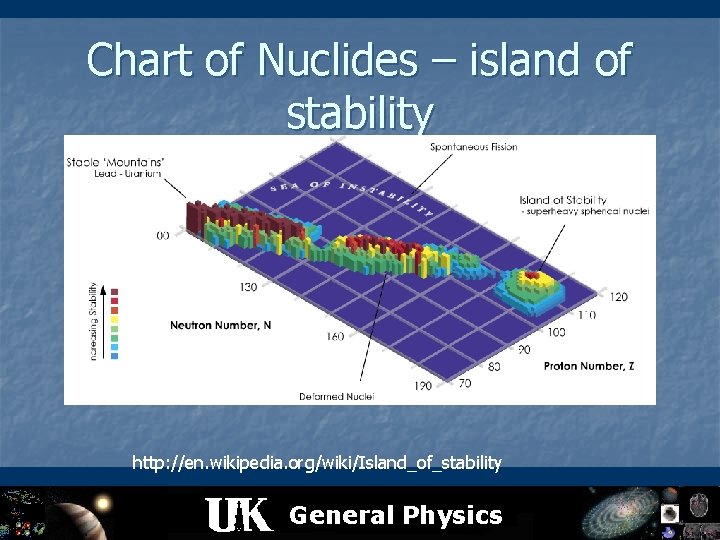
Chart of Nuclides – island of stability magic numbers http: //en. wikipedia. org/wiki/Island_of_stability General Physics

Alpha Decay n When a nucleus emits an alpha particle it loses two protons and two neutrons n n N decreases by 2 Z decreases by 2 A decreases by 4 Symbolically n n X is called the parent nucleus Y is called the daughter nucleus General Physics

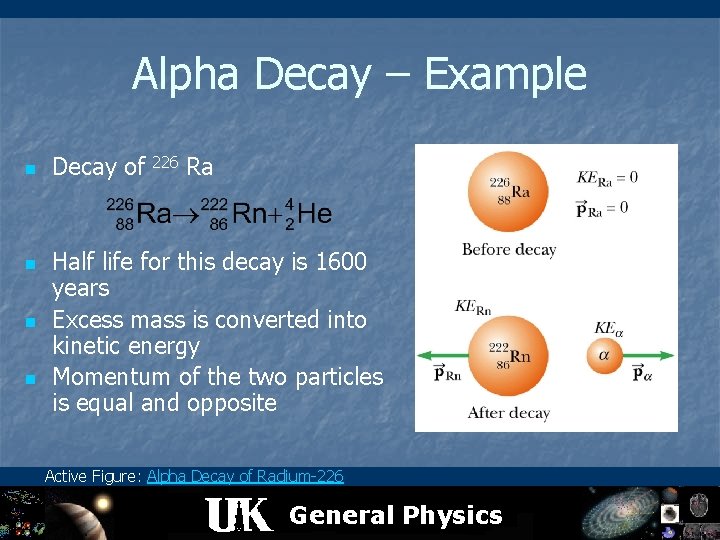
Alpha Decay – Example n n Decay of 226 Ra Half life for this decay is 1600 years Excess mass is converted into kinetic energy Momentum of the two particles is equal and opposite Active Figure: Alpha Decay of Radium-226 General Physics

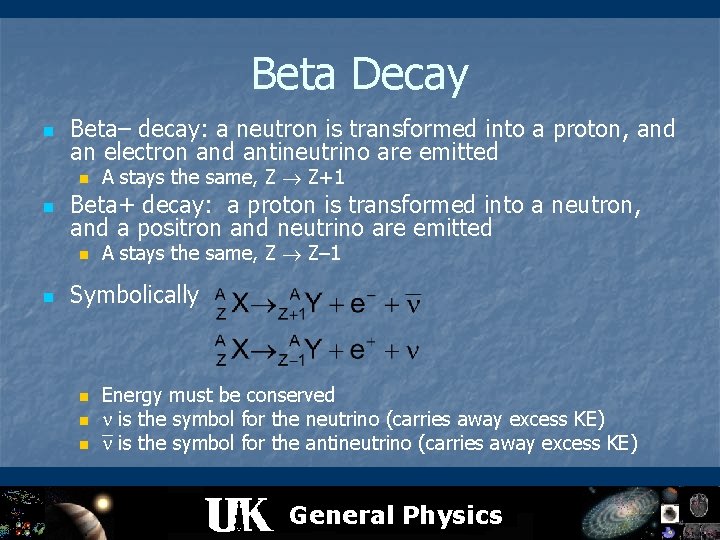
Beta Decay n Beta– decay: a neutron is transformed into a proton, and an electron and antineutrino are emitted n n Beta+ decay: a proton is transformed into a neutron, and a positron and neutrino are emitted n n A stays the same, Z Z+1 A stays the same, Z Z– 1 Symbolically n n n Energy must be conserved is the symbol for the neutrino (carries away excess KE) is the symbol for the antineutrino (carries away excess KE) General Physics

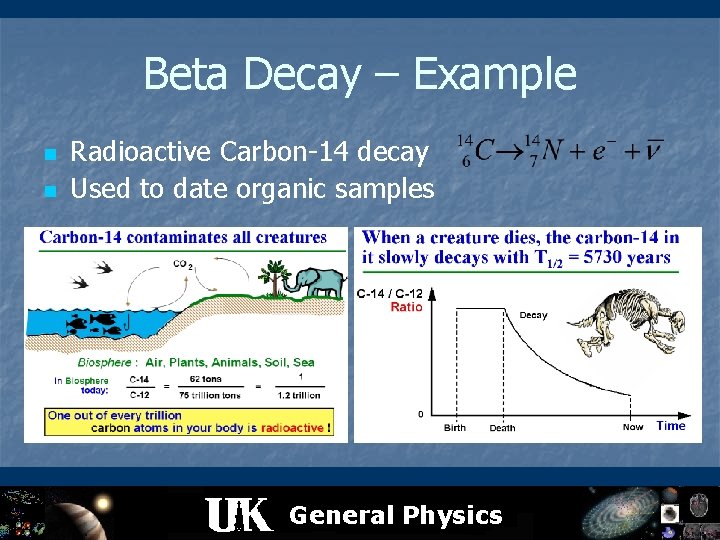
Beta Decay – Example n n Radioactive Carbon-14 decay Used to date organic samples General Physics

Gamma Decay n Gamma rays are given off when an excited nucleus “falls” to a lower energy state n n Similar to the process of electron “jumps” to lower energy states and giving off photons The photons are called gamma rays, very high energy relative to light The excited nuclear states result from “jumps” made by a proton or neutron The excited nuclear states may be the result of violent collision or more likely of an alpha or beta emission General Physics

Gamma Decay – Example n Example of a decay sequence n The first decay is a beta decay emission n The second step is a gamma decay emission n n C* indicates the Carbon nucleus is in an excited state Gamma emission doesn’t change either A or Z General Physics

Medical Applications of Radiation n Tracing n Radioactive particles can be used to trace chemicals participating in various reactions n n Example, 131 I to test thyroid action Sterilization n Radiation has been used to sterilize medical equipment Used to destroy bacteria, worms and insects in food Bone, cartilage, and skin used in graphs is often irradiated before grafting to reduce the chances of infection General Physics

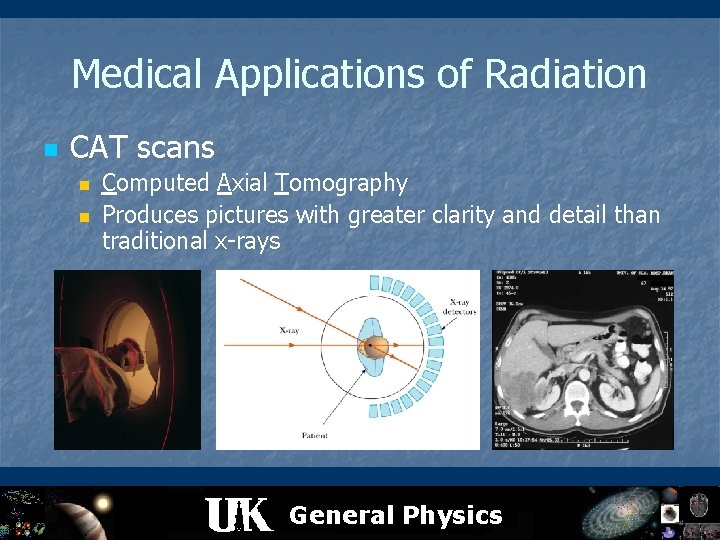
Medical Applications of Radiation n CAT scans n n Computed Axial Tomography Produces pictures with greater clarity and detail than traditional x-rays General Physics

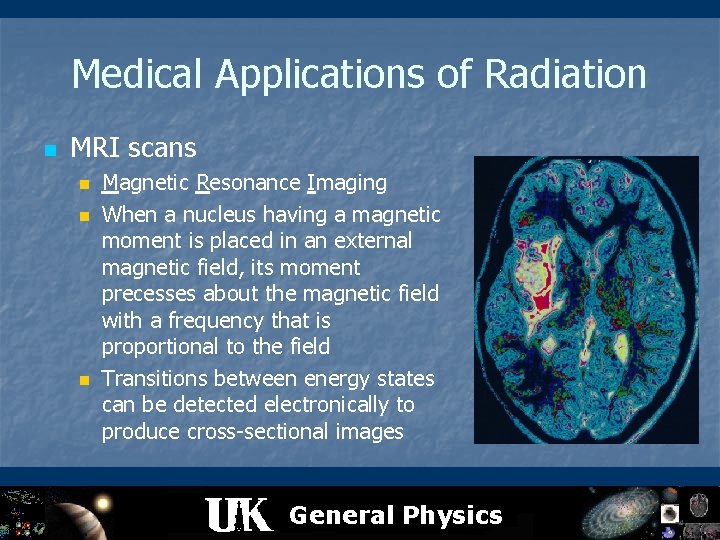
Medical Applications of Radiation n MRI scans n n n Magnetic Resonance Imaging When a nucleus having a magnetic moment is placed in an external magnetic field, its moment precesses about the magnetic field with a frequency that is proportional to the field Transitions between energy states can be detected electronically to produce cross-sectional images General Physics

Medical Applications of Radiation n 3 D-CRT Treatment n n Three-dimensional conformal radiation therapy uses sophisticated computers, CT scans and/or MRI scans to create detailed 3 -D representations of a tumor and surrounding organs Radiation beams are then shaped exactly to treat the size and shape of the tumor – nearby normal tissue receives less radiation exposure General Physics

3 D-CRT Treatment Planning General Physics