The Synthesis Purification and Characterization of Ferrocene Dr

- Slides: 24

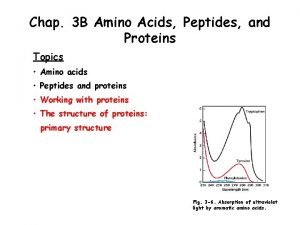
The Synthesis, Purification, and Characterization of Ferrocene Dr. Nisha Sharma

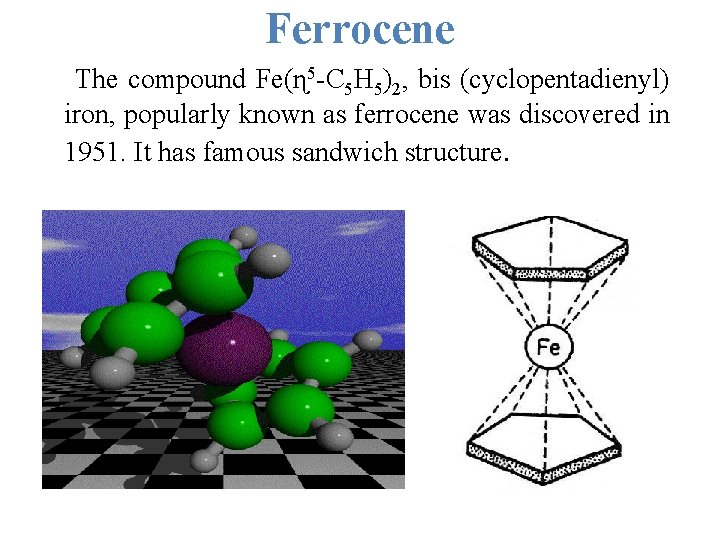
Ferrocene The compound Fe(ɳ 5 -C 5 H 5)2, bis (cyclopentadienyl) iron, popularly known as ferrocene was discovered in 1951. It has famous sandwich structure.

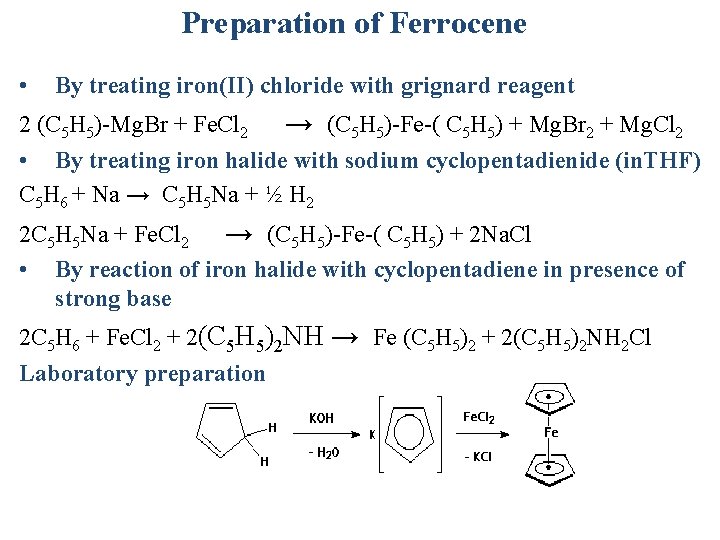
Preparation of Ferrocene • By treating iron(II) chloride with grignard reagent 2 (C 5 H 5)-Mg. Br + Fe. Cl 2 → (C 5 H 5)-Fe-( C 5 H 5) + Mg. Br 2 + Mg. Cl 2 • By treating iron halide with sodium cyclopentadienide (in. THF) C 5 H 6 + Na → C 5 H 5 Na + ½ H 2 2 C 5 H 5 Na + Fe. Cl 2 → (C 5 H 5)-Fe-( C 5 H 5) + 2 Na. Cl • By reaction of iron halide with cyclopentadiene in presence of strong base 2 C 5 H 6 + Fe. Cl 2 + 2(C 5 H 5)2 NH → Fe (C 5 H 5)2 + 2(C 5 H 5)2 NH 2 Cl Laboratory preparation

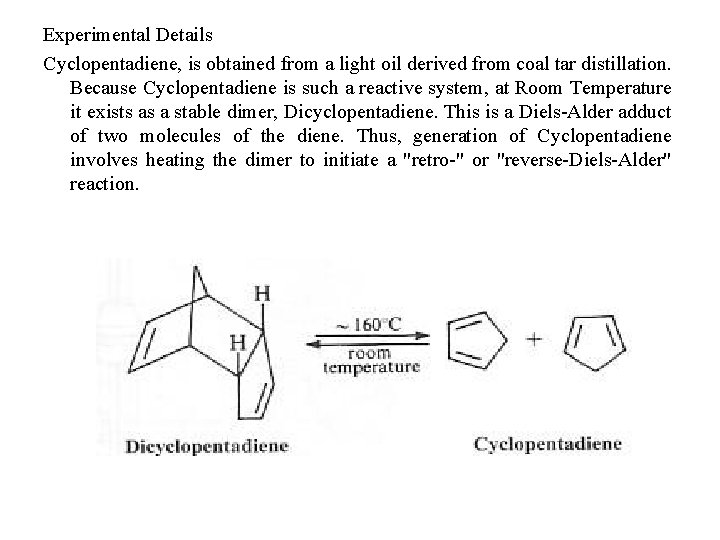
Experimental Details Cyclopentadiene, is obtained from a light oil derived from coal tar distillation. Because Cyclopentadiene is such a reactive system, at Room Temperature it exists as a stable dimer, Dicyclopentadiene. This is a Diels-Alder adduct of two molecules of the diene. Thus, generation of Cyclopentadiene involves heating the dimer to initiate a "retro-" or "reverse-Diels-Alder" reaction.

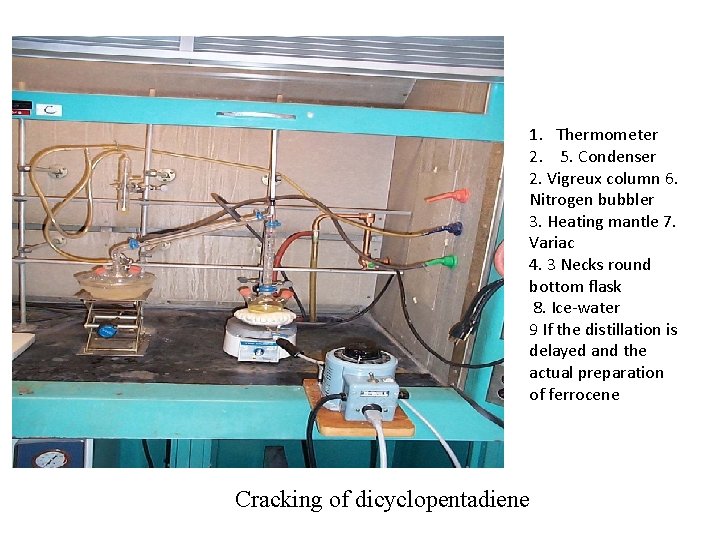
1. Thermometer 2. 5. Condenser 2. Vigreux column 6. Nitrogen bubbler 3. Heating mantle 7. Variac 4. 3 Necks round bottom flask 8. Ice-water 9 If the distillation is delayed and the actual preparation of ferrocene Cracking of dicyclopentadiene

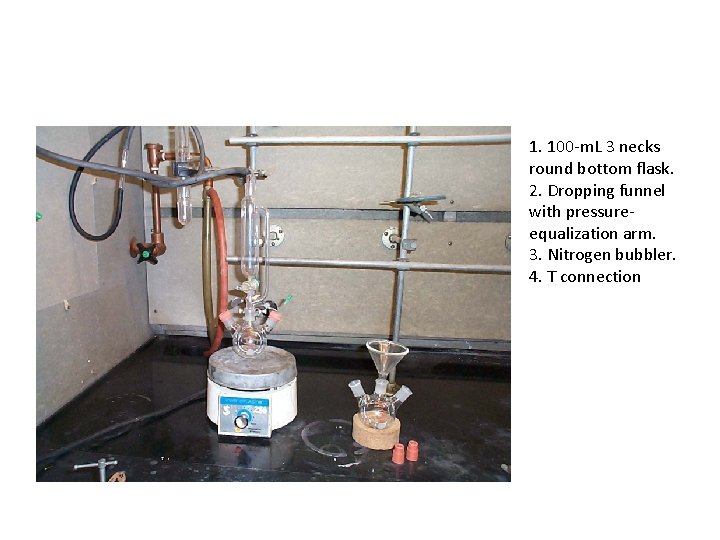
1. 100 -m. L 3 necks round bottom flask. 2. Dropping funnel with pressureequalization arm. 3. Nitrogen bubbler. 4. T connection

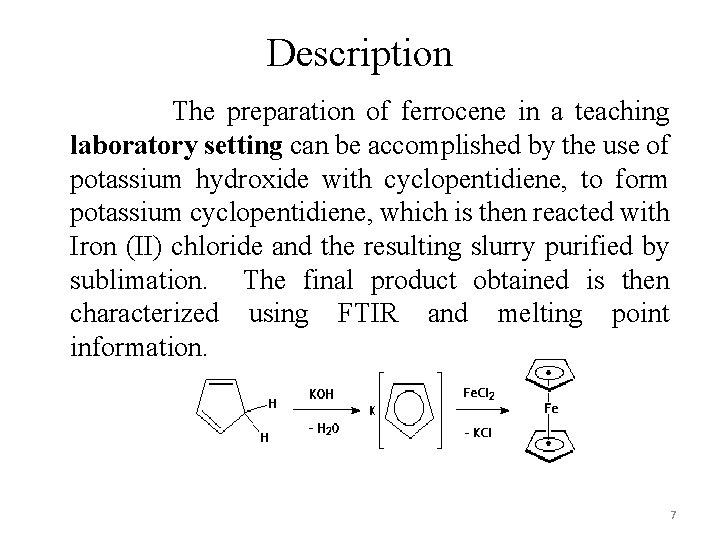
Description The preparation of ferrocene in a teaching laboratory setting can be accomplished by the use of potassium hydroxide with cyclopentidiene, to form potassium cyclopentidiene, which is then reacted with Iron (II) chloride and the resulting slurry purified by sublimation. The final product obtained is then characterized using FTIR and melting point information. 7

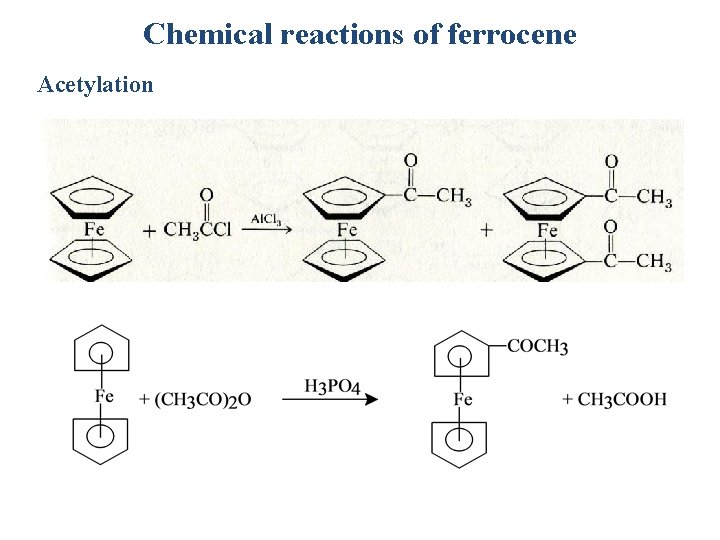
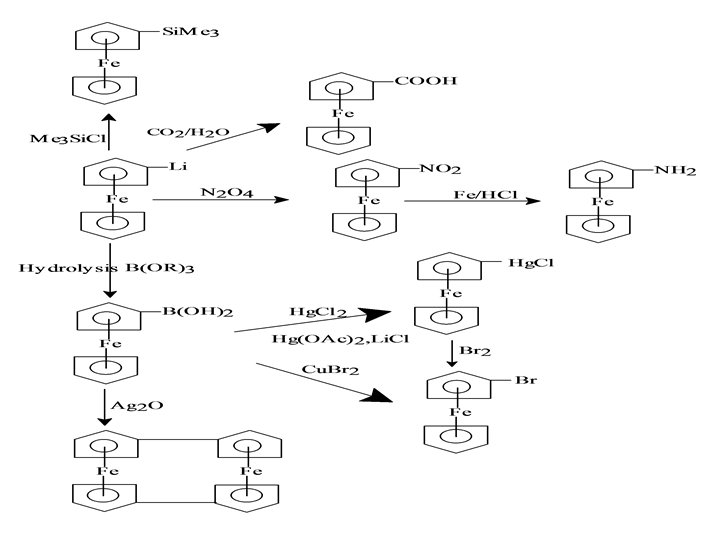
Chemical reactions of ferrocene Acetylation

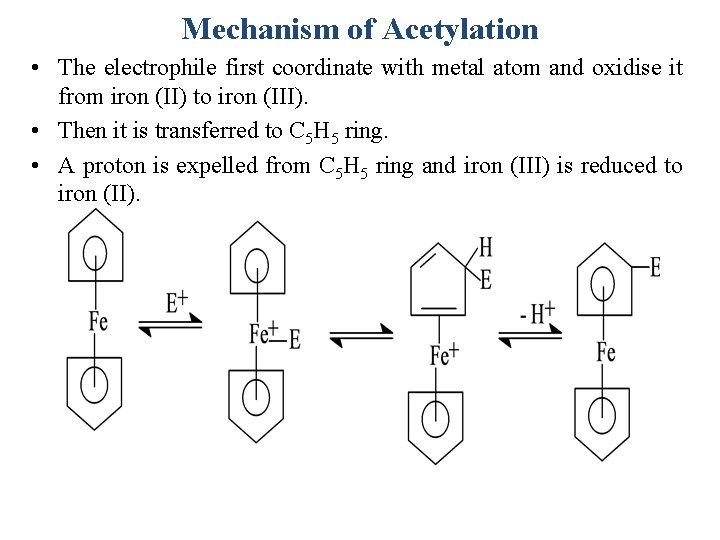
Mechanism of Acetylation • The electrophile first coordinate with metal atom and oxidise it from iron (II) to iron (III). • Then it is transferred to C 5 H 5 ring. • A proton is expelled from C 5 H 5 ring and iron (III) is reduced to iron (II).

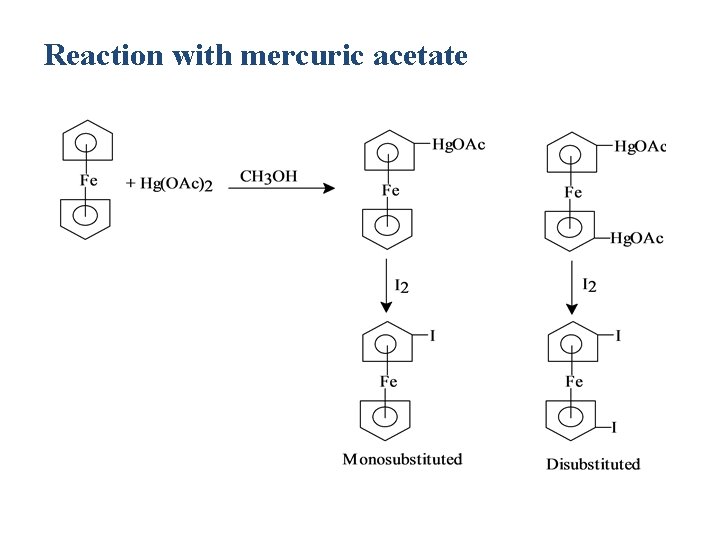
Reaction with mercuric acetate

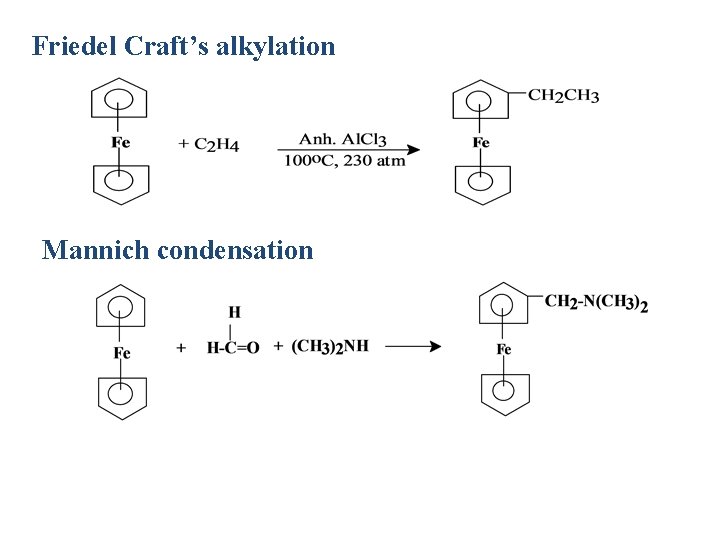
Friedel Craft’s alkylation Mannich condensation

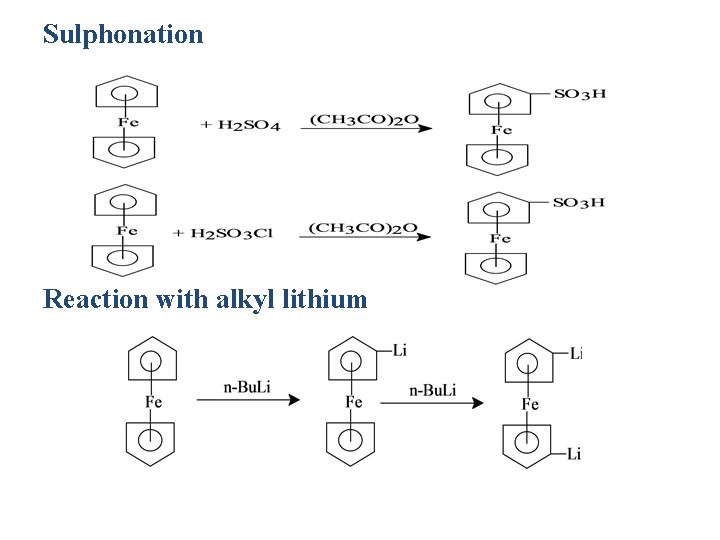
Sulphonation Reaction with alkyl lithium


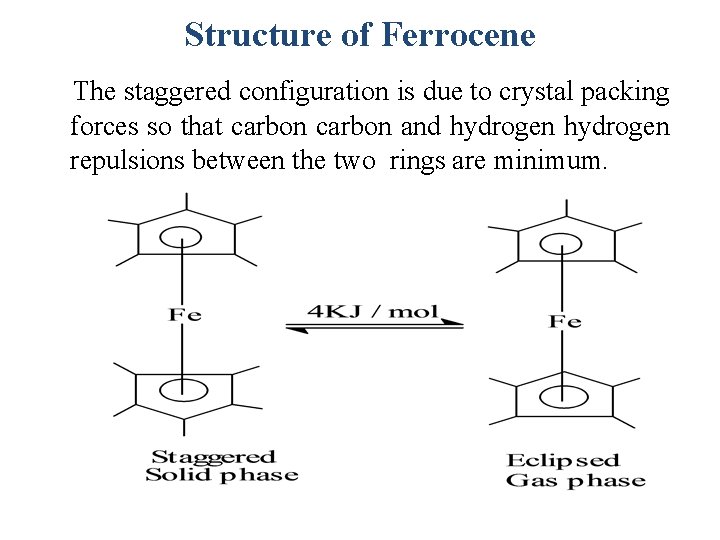
Structure of Ferrocene The staggered configuration is due to crystal packing forces so that carbon and hydrogen repulsions between the two rings are minimum.

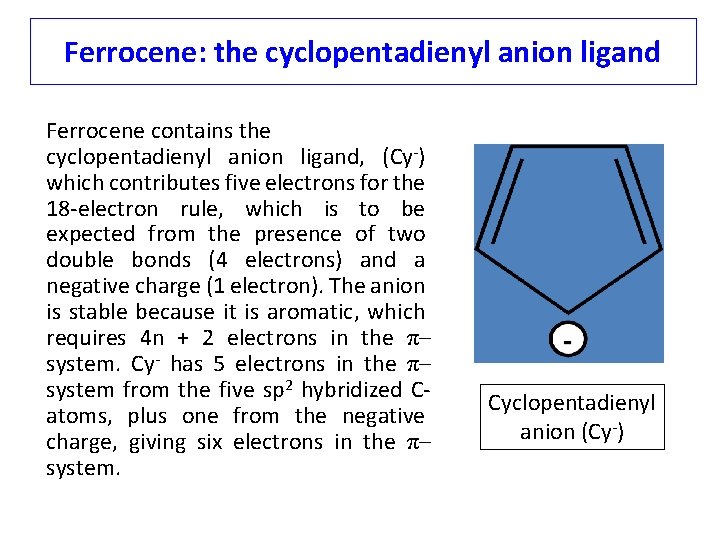
Ferrocene: the cyclopentadienyl anion ligand Ferrocene contains the cyclopentadienyl anion ligand, (Cy-) which contributes five electrons for the 18 -electron rule, which is to be expected from the presence of two double bonds (4 electrons) and a negative charge (1 electron). The anion is stable because it is aromatic, which requires 4 n + 2 electrons in the π– system. Cy- has 5 electrons in the π– system from the five sp 2 hybridized Catoms, plus one from the negative charge, giving six electrons in the π– system. Cyclopentadienyl anion (Cy-)

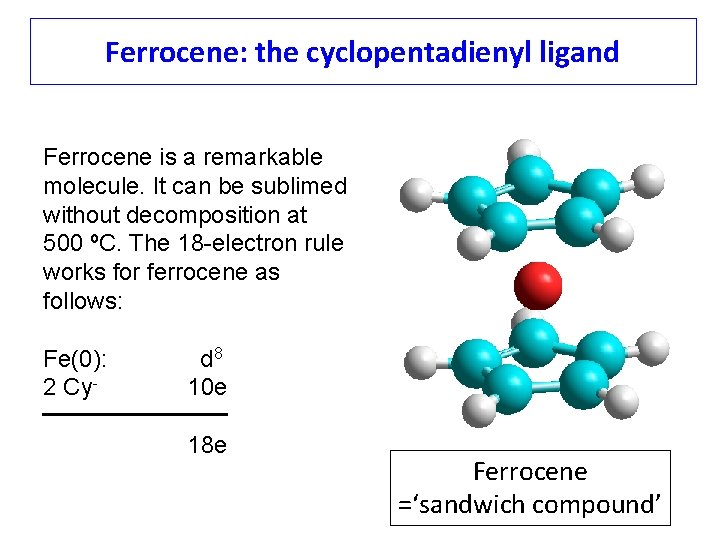
Ferrocene: the cyclopentadienyl ligand Ferrocene is a remarkable molecule. It can be sublimed without decomposition at 500 ºC. The 18 -electron rule works for ferrocene as follows: Fe(0): 2 Cy- d 8 10 e 18 e Ferrocene =‘sandwich compound’

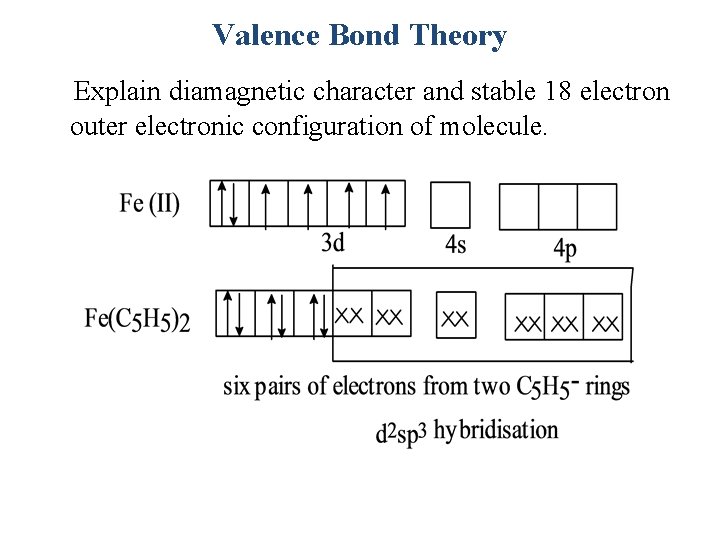
Valence Bond Theory Explain diamagnetic character and stable 18 electron outer electronic configuration of molecule.

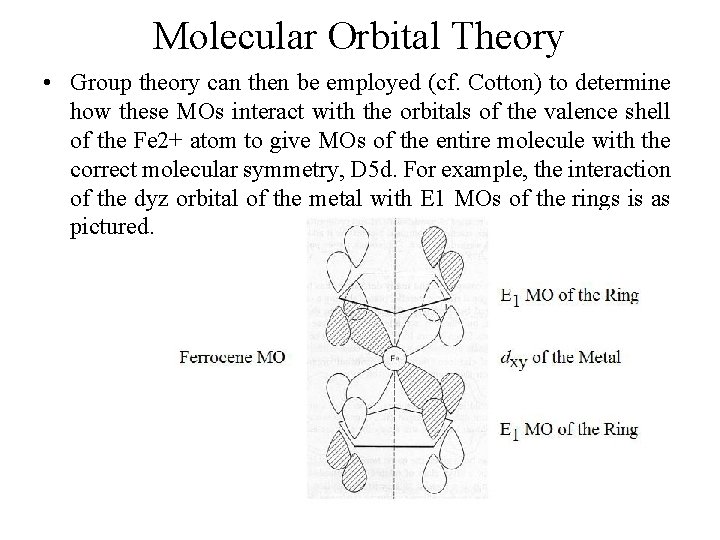
Molecular Orbital Theory • Group theory can then be employed (cf. Cotton) to determine how these MOs interact with the orbitals of the valence shell of the Fe 2+ atom to give MOs of the entire molecule with the correct molecular symmetry, D 5 d. For example, the interaction of the dyz orbital of the metal with E 1 MOs of the rings is as pictured.

Ferrocene is an extremely stable complex, stable in Air to temperatures of 500 o. C, because its 18 valence electrons occupy only bonding and non-bonding MOs. Twelve of these electrons are contributed to the structure by the cyclopentadienyl rings and occupy the lowest six Ferrocene MOs. The six electrons of the Fe 2+ contribute to the Ferrocene metal-like MOs. The strictly anti-bonding MOs of Ferrocene are not occupied


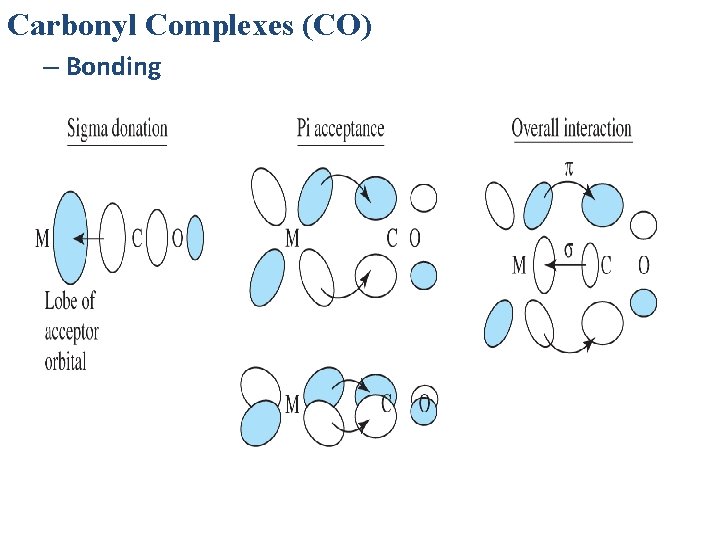
Carbonyl Complexes (CO) – Bonding

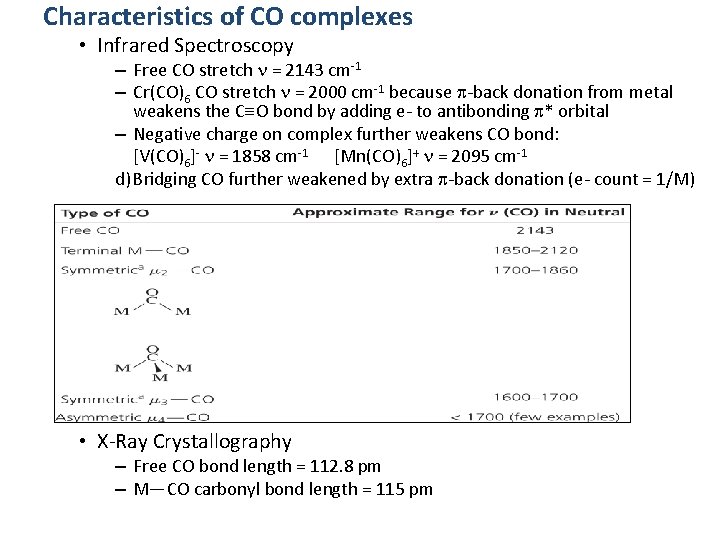
Characteristics of CO complexes • Infrared Spectroscopy – Free CO stretch n = 2143 cm-1 – Cr(CO)6 CO stretch n = 2000 cm-1 because p-back donation from metal weakens the C O bond by adding e- to antibonding p* orbital – Negative charge on complex further weakens CO bond: [V(CO)6]- n = 1858 cm-1 [Mn(CO)6]+ n = 2095 cm-1 d) Bridging CO further weakened by extra p-back donation (e- count = 1/M) • X-Ray Crystallography – Free CO bond length = 112. 8 pm – M—CO carbonyl bond length = 115 pm

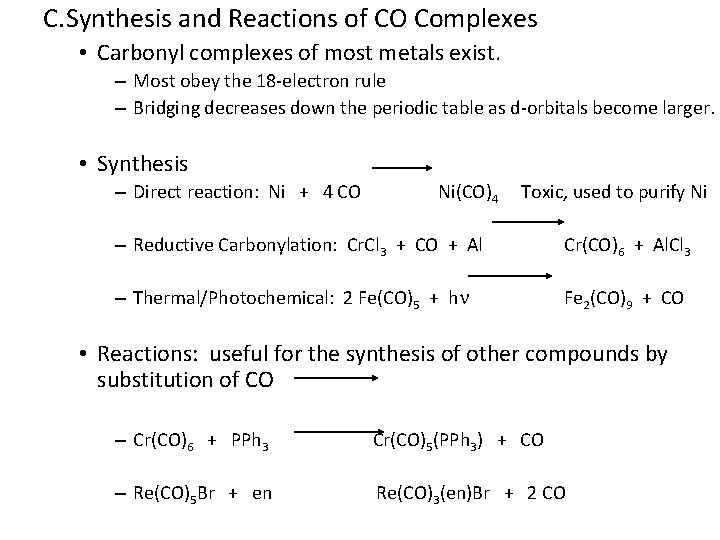
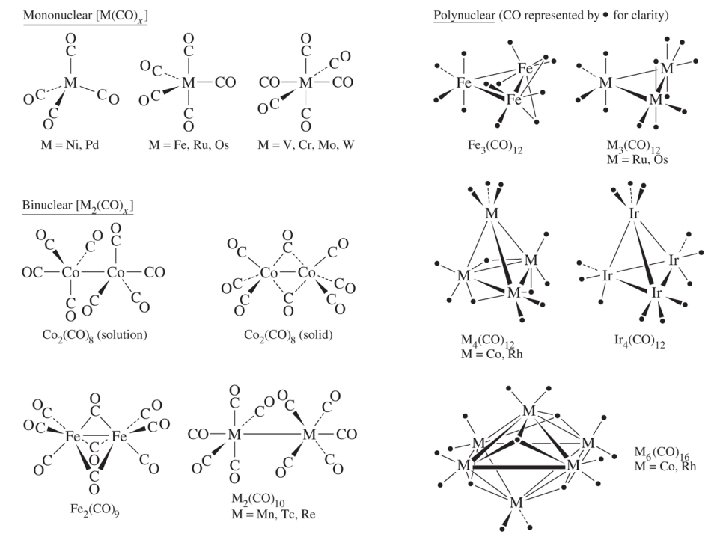
C. Synthesis and Reactions of CO Complexes • Carbonyl complexes of most metals exist. – Most obey the 18 -electron rule – Bridging decreases down the periodic table as d-orbitals become larger. • Synthesis – Direct reaction: Ni + 4 CO Ni(CO)4 Toxic, used to purify Ni – Reductive Carbonylation: Cr. Cl 3 + CO + Al Cr(CO)6 + Al. Cl 3 – Thermal/Photochemical: 2 Fe(CO)5 + hn Fe 2(CO)9 + CO • Reactions: useful for the synthesis of other compounds by substitution of CO – Cr(CO)6 + PPh 3 Cr(CO)5(PPh 3) + CO – Re(CO)5 Br + en Re(CO)3(en)Br + 2 CO

 Protein characterization methods
Protein characterization methods Purification and characterization of organic compounds
Purification and characterization of organic compounds Ferrocene
Ferrocene Coordination isomerism
Coordination isomerism Net3 chemistry
Net3 chemistry Acetylferrocene ir spectrum labeled
Acetylferrocene ir spectrum labeled Synthesis and characterization of aspirin
Synthesis and characterization of aspirin Define indirect characterization
Define indirect characterization Indirect characterization example
Indirect characterization example Need of water purification
Need of water purification Double pot method for disinfection of wells
Double pot method for disinfection of wells Introduction of purification of water
Introduction of purification of water Purification adn sur colonne de silice
Purification adn sur colonne de silice Double pot method water purification
Double pot method water purification Purification of benzaldehyde
Purification of benzaldehyde Enzyme purification
Enzyme purification Gel filtration
Gel filtration Inert gas purification
Inert gas purification Purification of citric acid
Purification of citric acid Edman degradation steps
Edman degradation steps Salting out protein purification
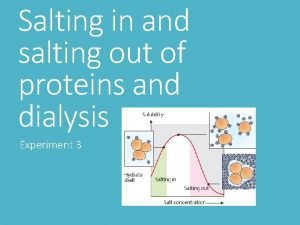
Salting out protein purification Inert gas purification
Inert gas purification Salting out proteins
Salting out proteins Dna purification overview
Dna purification overview Violet
Violet