Synthesis and characterization of ferrocene derivatives with extended




- Slides: 7

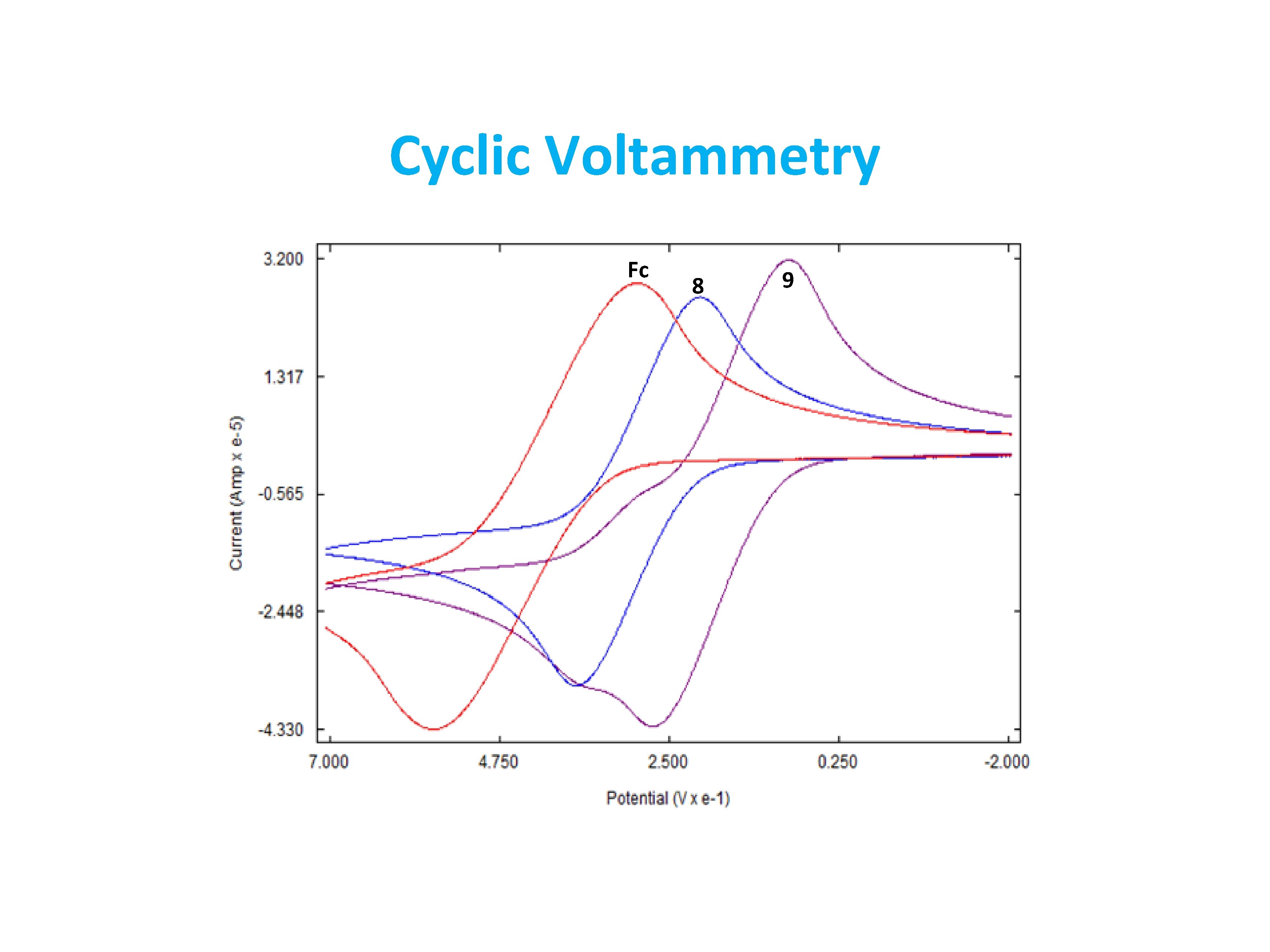
Synthesis and characterization of ferrocene derivatives with extended carbocyclic rings Stone Naquin, Aaron Naquin, Gwyneth Engeron, Derek Daigle, Mary Lo, Frank Fronczek, and Uttam Pokharel Department of Physical Sciences, Nicholls State University, Thibodaux, LA 70301 Introduction In this project, we explore the functionalization of ferrocene with carbocyclic rings. Although the chemistry of ferrocene and its derivatives have been explored from several decades, the derivatives with extended carbocyclic rings have not been fully studied yet. The long-term goal of our research group is to develop a facile methodology of extending the π-conjugation of ferrocene. In this process, we have synthesized various carbocyclic rings to the ferrocene moiety which will be discussed in this presentation. Results • • Friedel-Crafts acylation reaction between ferrocene and succinic anhydride gives a mixture of (3 -carboxypropionyl)ferrocene, 1 and 1, 1’-1 -(3 -carboxypropionyl)ferrocene, 2 The mixture of these two carboxylic acids are separated by taking advantage of their inequal solubility in hot water. • The two diasteromers 6 and 7 were separated by silica gel column chromatography using a mixture of ethyl acetate and dichloromethane (2: 1) • The meso isomer, 6 is light yellow while the racemic mixture, 7 appeared as dark red powder • Electrochemistry was investigated in dichloromethane using Ag/Ag. Cl as reference electrode, Pt wire as counter electrode and glassy carbon as working electrode • The oxidation potential of the iron (II) center reduces with increasing number of methylene groups in the Cp ring • Oxidation potential of 9 is less than 8 by 0. 22 V. Both complexes exhibited less oxidation potential than ferrocene. Conclusions • We synthesized and fully characterized the bicyclic and tetracyclic derivatives of ferrocene • We separated two diasteromers 6 and 7 and assigned their configurations • The oxidation potential of the tetramethylene ferrocene derivative is higher than that of octamethylene derivative by 0. 22 V. Acknowledgements • Financial support • Louisiana Board of Regents • Department of Chemistry and Physical Sciences, Nicholls State University • • • The reduction was monitored by the color change of compounds from deep red to bright yellow. Loss of carbonyl stretching was observed in IR spectroscopy The 1 H NMR showed distinctive signals for three CH 2 groups • The diastereomeric mixture of 6 and 7 gave a single product, 9 as a viscous liquid • 1 H NMR of 9 showed only 6 signals (one triplet, one doublet and three multiplets) showing high symmetry in the molecule. • The exo and endo hydrogens of the six member rings are diastereotopic giving different signals in NMR References Maharjan, B. L. , Ferrocene-Fused Derivatives of Acenes, Tropones, and Thiepins. In Ph. D dissertation, University of Kentucky: 2015.

Friedel-Crafts Acylation

Clemmensen Reduction

Ring-Closing

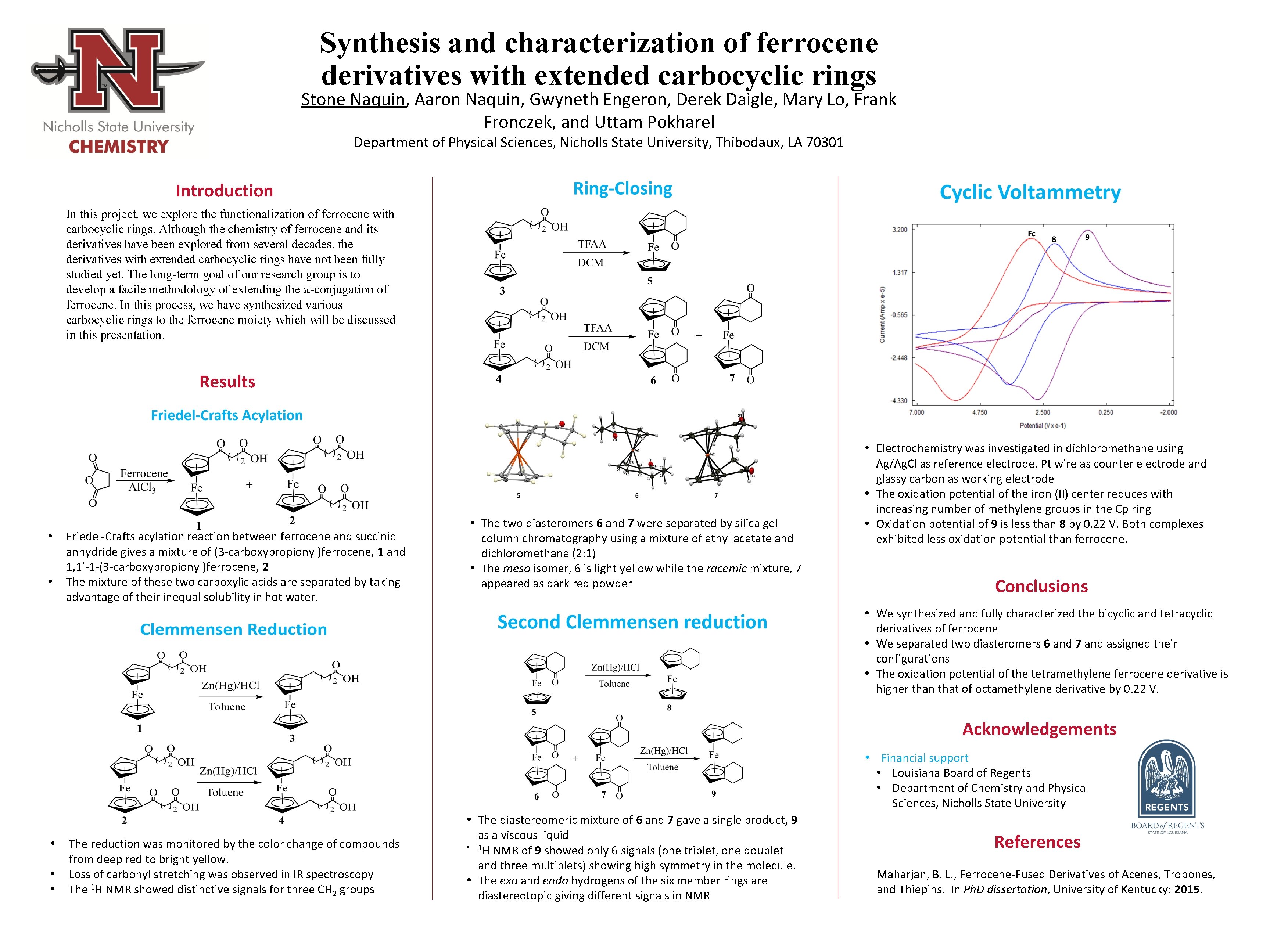
5 6 7

Second Clemmensen reduction

Cyclic Voltammetry Fc 8 9










