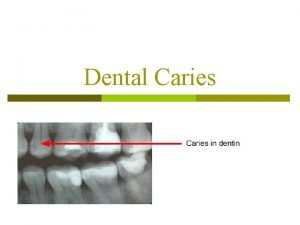
The role of fluoride in dental caries prevention














- Slides: 31

The role of fluoride in dental caries prevention Dr Ahmad Aljafari BDS, MFDS RCSEd, MSc, Ph. D

Lecture outline § Fluorine in nature § History of fluoride use in dentistry § § Fluoride’s mechanism of action in caries prevention Overview of methods for fluoride delivery

Fluorine in nature

Fluorine Fluorine (F), has an atomic number of 9. Part of the halogen group. At room temperature, it is a gas of diatomic molecules (F 2) The gas is pale, yellowgreen, pungent, and poisonous. Used in aluminum refining, refrigerants and cookware manufacturing, and pharmaceuticals.

Fluorine in nature The 24 th most abundant element in the universe. The 13 th in earth crust. Highly reactive. Hence, combines with other elements (e. g. , calcium, sodium) in nature and is found only in mineral form. Fluorite (Ca. F 2) is the primary mineral source of fluorine, although other forms, such as fluorapatite (Ca 5(PO 4)3 F) and cryolite (Na 3 Al. F 6) are also used. Fluorite

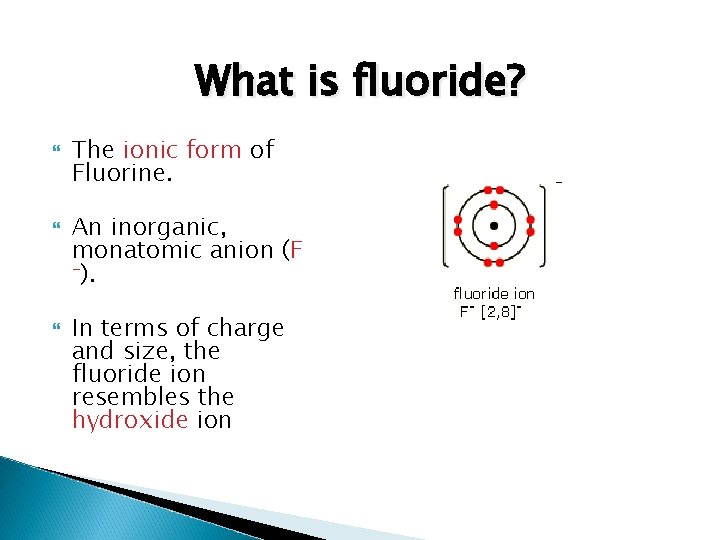
What is fluoride? The ionic form of Fluorine. An inorganic, monatomic anion (F -). In terms of charge and size, the fluoride ion resembles the hydroxide ion

Fluoride in nature § Mineral form in earth crust (e. g. fluorite). § Seawater (1. 1 ppm). § Fresh water (highly variable). § Fish (0. 2 -0. 4 mg/100 g). § Tea: (0. 1 – 0. 6 mg/100 ml). § Other foods might contain fluoride in very low concentrations.

History of fluoride in dentistry

1874: Carl Erhadt suggested potassium fluoride supplements to preserve teeth. 1892: Sir James Crichton Browne noted an increased susceptibility to caries when switching from brown (higher in Fluoride) to white bread Fluoride supplement leaflet in 1902 (Pindborg 1965)

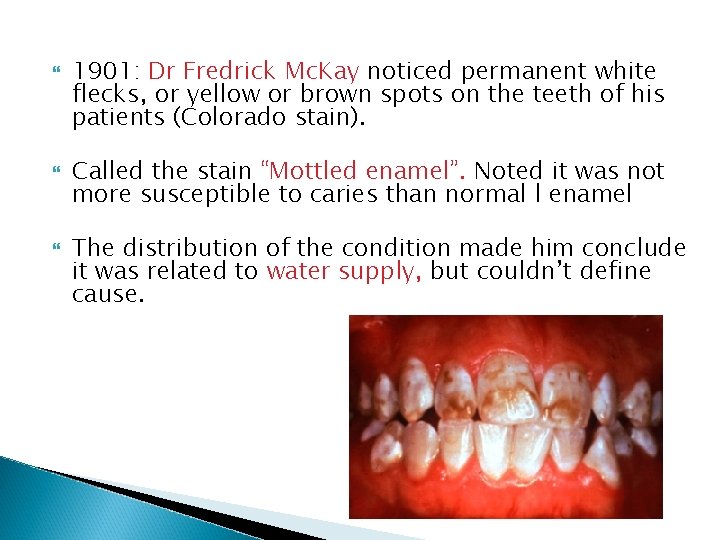
1901: Dr Fredrick Mc. Kay noticed permanent white flecks, or yellow or brown spots on the teeth of his patients (Colorado stain). Called the stain “Mottled enamel”. Noted it was not more susceptible to caries than normal l enamel The distribution of the condition made him conclude it was related to water supply, but couldn’t define cause.

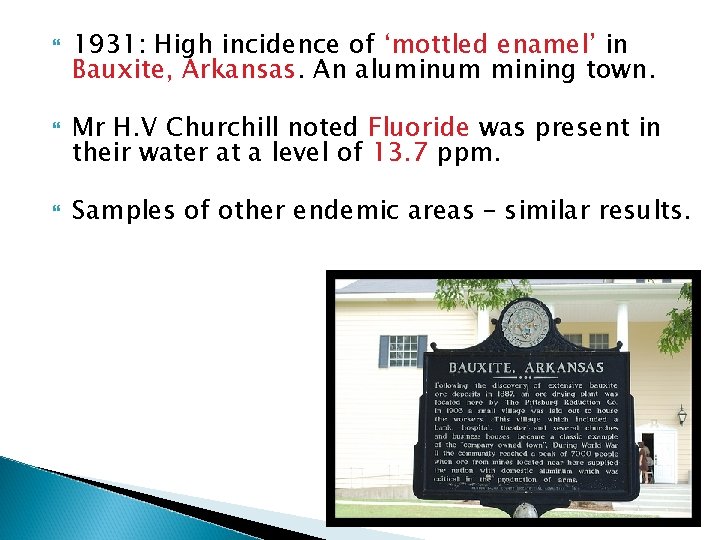
1931: High incidence of ‘mottled enamel’ in Bauxite, Arkansas. An aluminum mining town. Mr H. V Churchill noted Fluoride was present in their water at a level of 13. 7 ppm. Samples of other endemic areas – similar results.

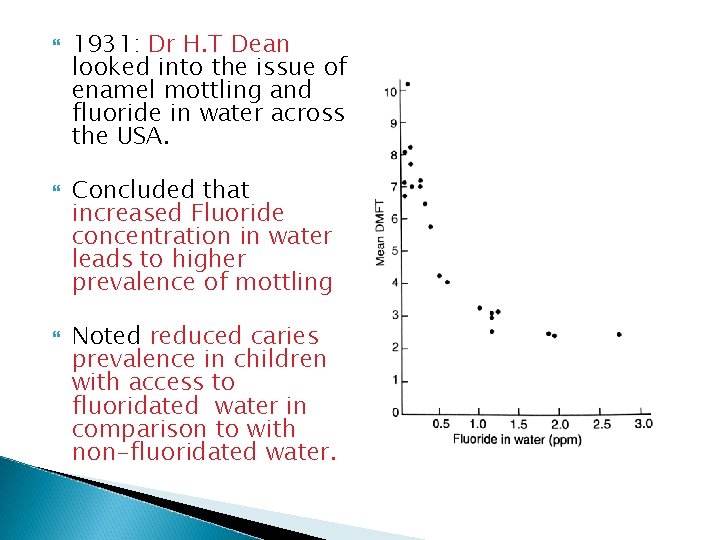
1931: Dr H. T Dean looked into the issue of enamel mottling and fluoride in water across the USA. Concluded that increased Fluoride concentration in water leads to higher prevalence of mottling Noted reduced caries prevalence in children with access to fluoridated water in comparison to with non-fluoridated water.

Fluoride’s mechanism of action in caries prevention

Mechanism of Action Pre-eruptive (Systemic): Less important Post-eruptive (Topical): More important

Pre-eruptive (systemic) q Used to be the focus of research prior to the 1980 s. q Nowadays we know that its impact is minimal. It is insufficient for caries prevention.

Pre-eruptive (systemic) v Earlier q work suggested that: It improves tooth morphology : ◦ More rounded cusps. ◦ Shallower inclines. ◦ More favourable fissure patterns. q Is incorporated into enamel to make it more resistant to the demineralization process. v More recent work demonstrated that fluoride incorporated during tooth development does not reduce solubility.

Post-eruptive (topical) q The relevant mode of action nowadays. q The outcome is the result of three processes: 1. Reduction of susceptibility to demineralization. 2. Encouragement of enamel remineralization. 3. Inhibition of cariogenic bacteria metabolism.

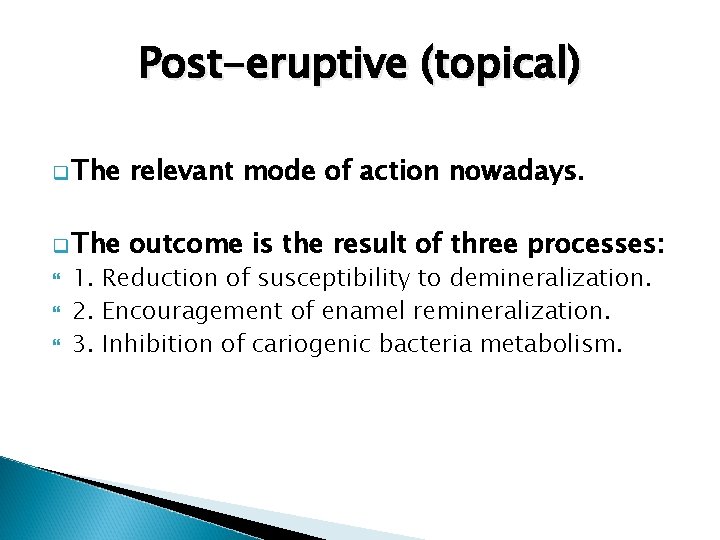
Reduction of susceptibility to demineralization Enamel is constituted of 95% mineral, 4% water, and 1% protein and lipid. The minerals form hydroxyapatite crystals (Ca 10(PO 4)6 OH 2). The crystals form enamel rods extending from the DEJ to the surface Robinson (2009)

Reduction of susceptibility to demineralization § § Conditions during tooth development and after formation frequently lead to mineral substitutions within the crystals. Ions such as carbonate and magnesium tend to replace calcium in the crystals. This disrupts crystal structure. In turn, this facilitates demineralization upon acid attacks and makes remineralization more difficult.

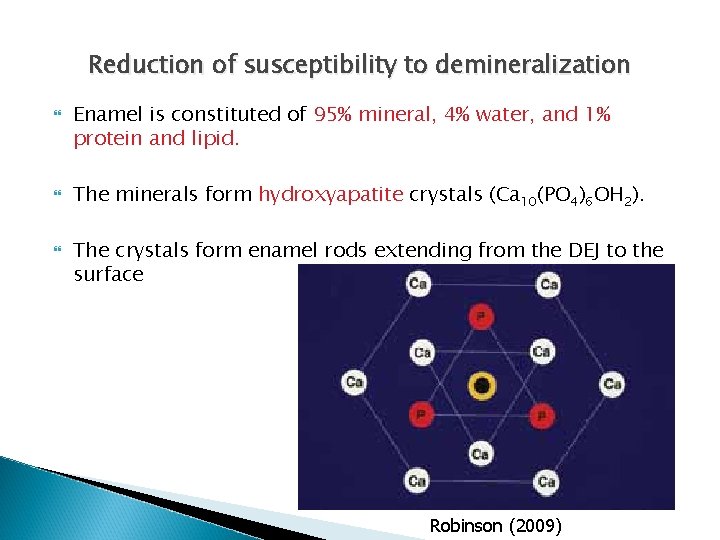
Reduction of susceptibility to demineralization In the presence of Fluoride surrounding enamel, it replaces the hydroxyl ion (OH-) Substitution occurs mostly on enamel surface (5 -10 µm). New crystals formed are fluorapatite (Ca 10(PO 4)6 F 2). (Posner 1985)

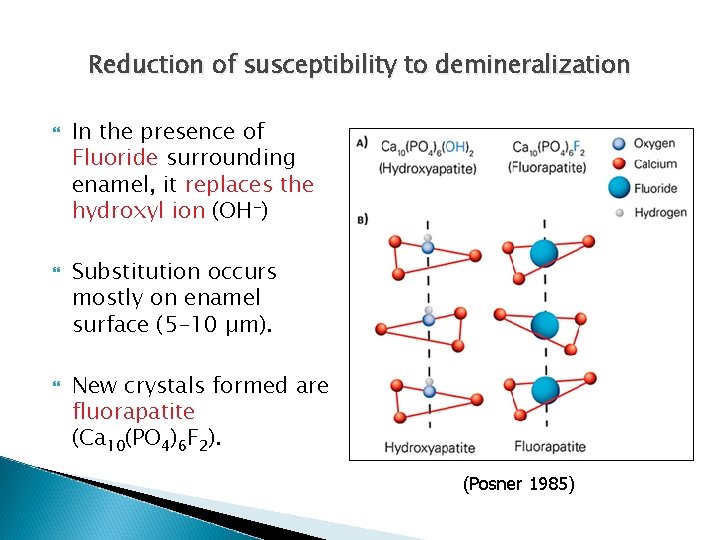
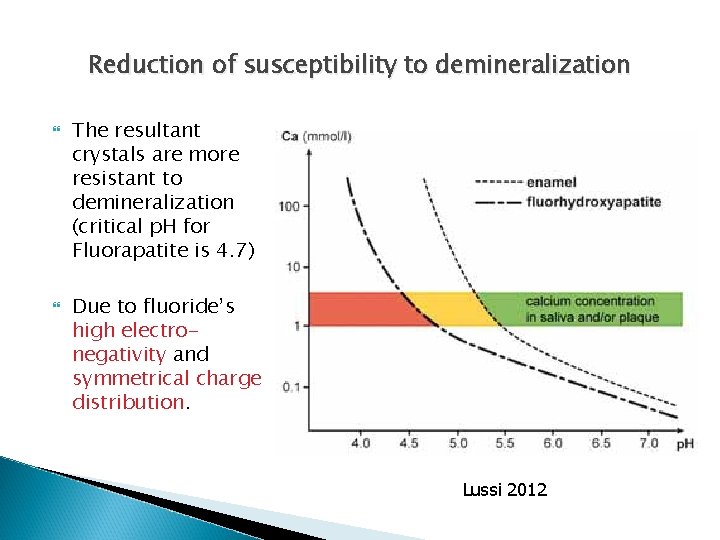
Reduction of susceptibility to demineralization The resultant crystals are more resistant to demineralization (critical p. H for Fluorapatite is 4. 7) Due to fluoride’s high electronegativity and symmetrical charge distribution. Lussi 2012

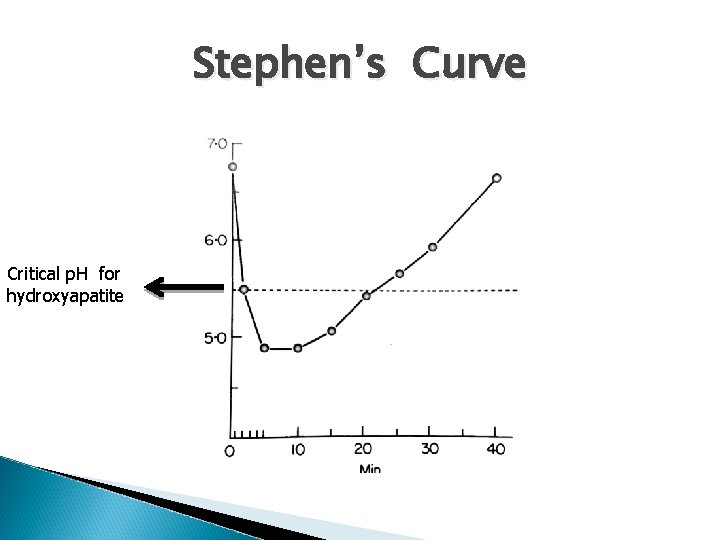
Encouragement of enamel remineralization At a p. H of 7, calcium and phosphate ions in the enamel and the surrounding plaque fluid are in an equilibrium. Acids produced by cariogenic bacteria in the dental plaque mean there is a release of H+ and a drop in p. H. H+ decreases the OH– concentration and interacts with the phosphate ions in the plaque fluid. At a p. H of 5. 5 (critical p. H), the calcium and phosphate ions concentrations in the plaque fluid are not sufficient to maintain the enamel in a stable equilibrium and hydroxyapatite crystals start to dissolve.

Stephen’s Curve Critical p. H for hydroxyapatite

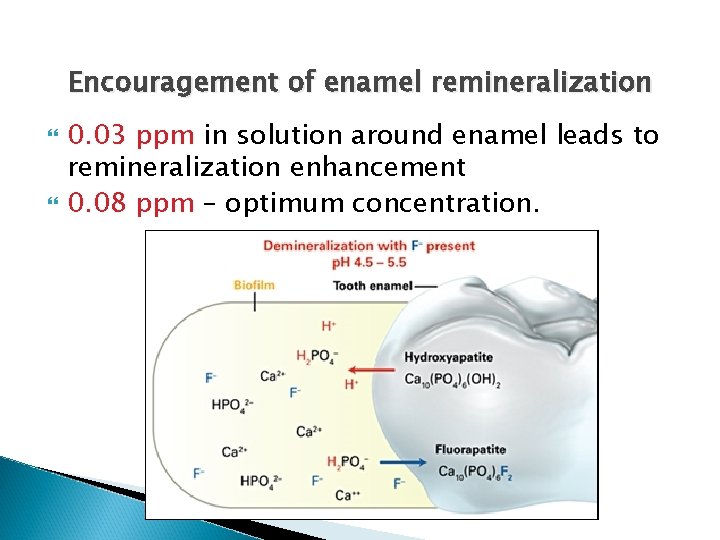
Encouragement of enamel remineralization During remineralization, calcium and phosphate ions move from the supersaturated plaque fluid to the enamel. When fluoride is present in the plaque fluid, it gets absorbed to the enamel crystals and attracts the calcium and phosphate ions. It also reduces the uptake of carbonate. The resultant crystals (fluorapatite) are less soluble.

Encouragement of enamel remineralization 0. 03 ppm in solution around enamel leads to remineralization enhancement 0. 08 ppm – optimum concentration.

Inhibition of cariogenic bacteria metabolism Fluoride in its ionic form is unable to cross the cell membrane. In a lower p. H some of the fluoride becomes in the form of hydrofluoric acid (HF). HF can rapidly diffuse into cariogenic bacterial cells. Inside the cell, HF dissolves back to H+ and F-

Inhibition of cariogenic bacteria metabolism q Fluoride presents an antimicrobial effect in two possible mechanisms: q q Interacting with the enzyme enolase to reduce acid production directly. Interacting with phosphotransferase system (PTS) pathway to decrease the amount of sugar entering the cell reducing acid production indirectly. The H+ accompanying the fluoride into the cell causes over-acidification of the cytoplasm. which can also inhibit the mechanism of glucose transport into the cell.

Overview of methods for fluoride delivery

Overview of methods for fluoride delivery q The ideal method to deliver fluoride should: Provide a long-term source for fluoride in the solution surrounding the enamel. Lead to only a minimal amount of fluoride being systemically ingested. Be cost-effective.

Overview of methods for fluoride delivery q Fluoride supplements: Drops Tablets q Fluoridated water q Fluoridated foods (salt, milk) q Home applied fluoride: Toothpaste Mouthwash q Fluoride in dental materials q Professionally applied fluoride Gels Varnish Slow release devices

Thank you
 Primary prevention secondary prevention tertiary prevention
Primary prevention secondary prevention tertiary prevention Finns modification of cavity preparation
Finns modification of cavity preparation Forward caries definition
Forward caries definition Gj mount classification of caries
Gj mount classification of caries Dentobuff strip test
Dentobuff strip test Odontoclasia meaning
Odontoclasia meaning Soft caries definition
Soft caries definition Turbid dentin in carious tooth
Turbid dentin in carious tooth Hopewood house study
Hopewood house study Histopathology of enamel caries
Histopathology of enamel caries Dental caries
Dental caries Argon fluoride laser to practical fusion
Argon fluoride laser to practical fusion Plumbic fluoride formula
Plumbic fluoride formula Positive negative attract each other
Positive negative attract each other Fluoride ion
Fluoride ion Shoe leather survey fluoride
Shoe leather survey fluoride Palladium (iv) oxide formula
Palladium (iv) oxide formula Chapter 15 preventive dentistry
Chapter 15 preventive dentistry Fluorapatite vs hydroxyapatite
Fluorapatite vs hydroxyapatite Silver diamine fluoride brighton
Silver diamine fluoride brighton Cld of fluoride
Cld of fluoride Lithium and fluoride compound name
Lithium and fluoride compound name Fluoridation of water pros and cons
Fluoridation of water pros and cons Co lewis structure
Co lewis structure Lithium oxide ionic bonding diagram
Lithium oxide ionic bonding diagram Aluminium fluoride market
Aluminium fluoride market Activadr
Activadr Knutson's technique
Knutson's technique How many electrons in each shell bohr diagram
How many electrons in each shell bohr diagram Donora fluoride fog
Donora fluoride fog Fluoride vs fluorine
Fluoride vs fluorine Kf lewis dot
Kf lewis dot