The HartreeFockRoothaan Method Variational Principle Energy of a





- Slides: 16

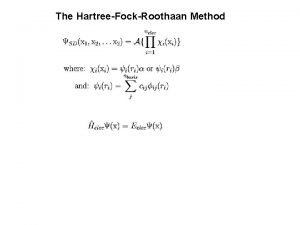
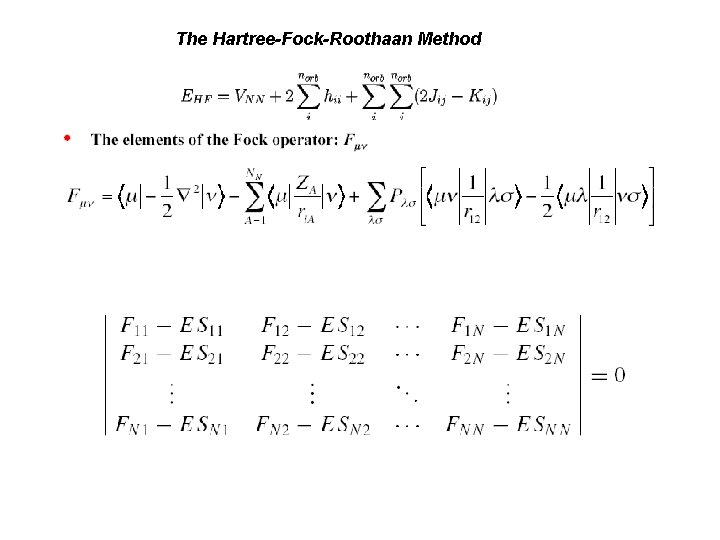
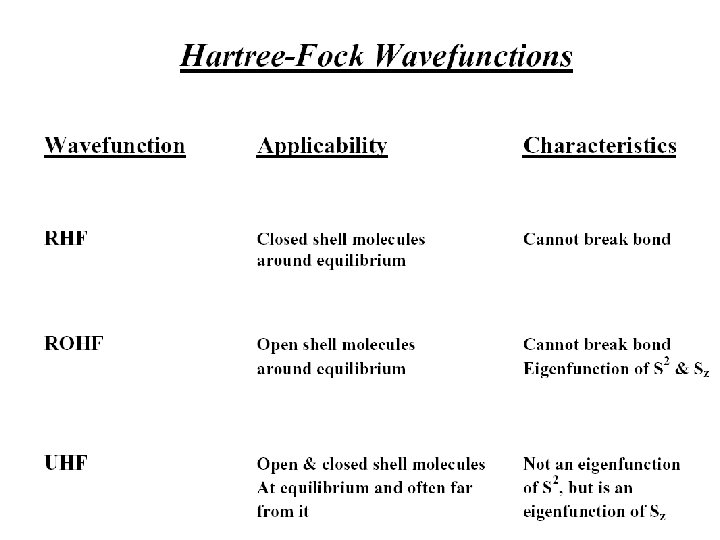
The Hartree-Fock-Roothaan Method

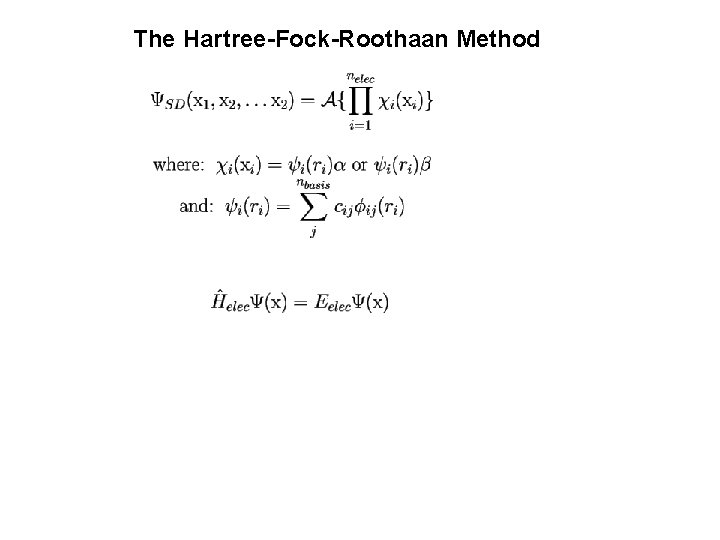
Variational Principle

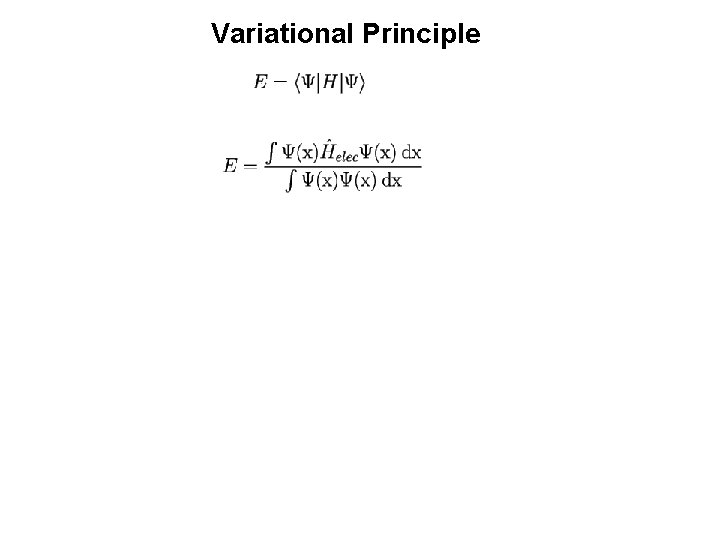
Energy of a Slater Determinant Where: And the Hamiltonian operator is given by:

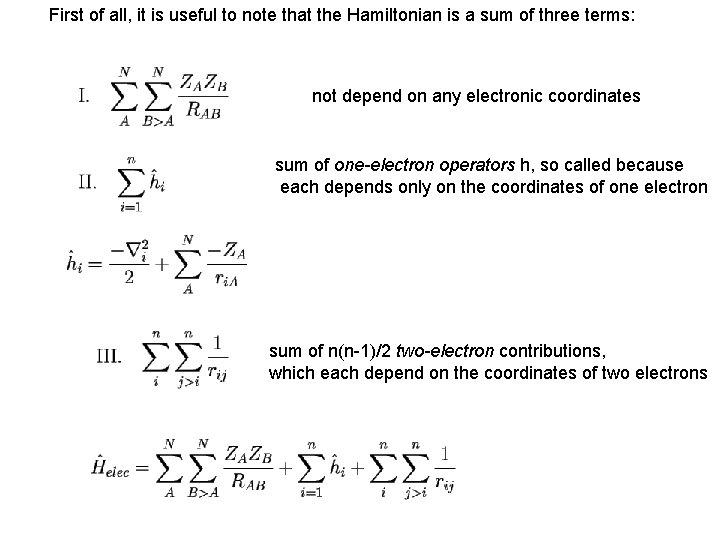
First of all, it is useful to note that the Hamiltonian is a sum of three terms: not depend on any electronic coordinates sum of one-electron operators h, so called because each depends only on the coordinates of one electron sum of n(n-1)/2 two-electron contributions, which each depend on the coordinates of two electrons

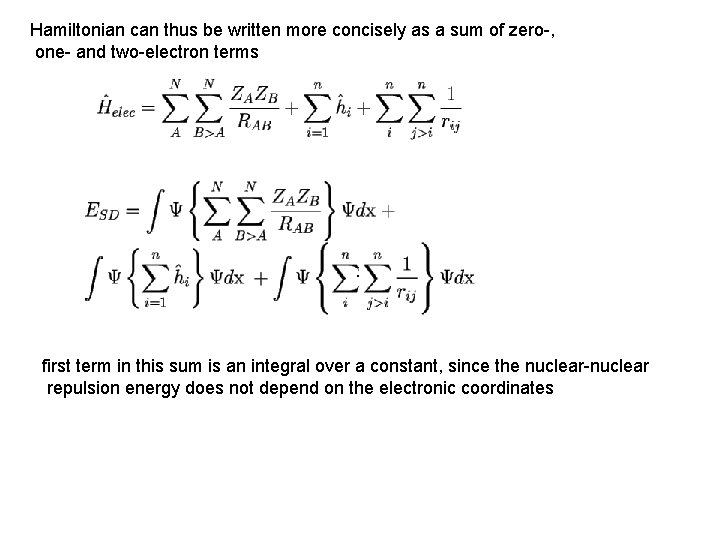
Hamiltonian can thus be written more concisely as a sum of zero-, one- and two-electron terms : first term in this sum is an integral over a constant, since the nuclear-nuclear repulsion energy does not depend on the electronic coordinates

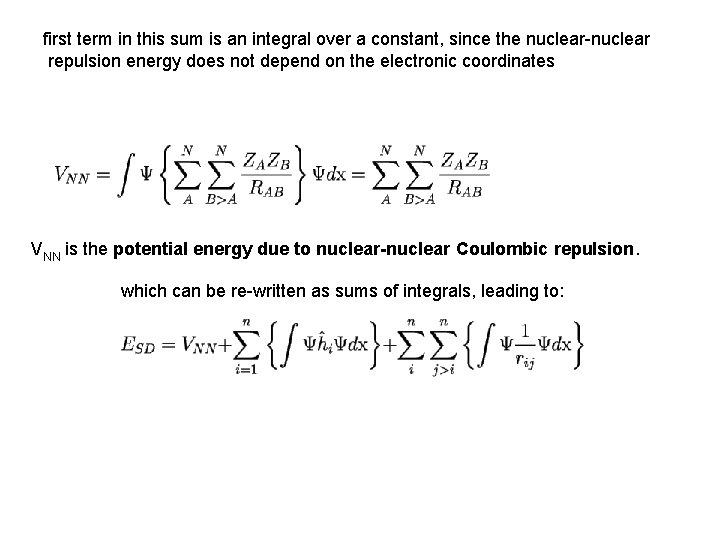
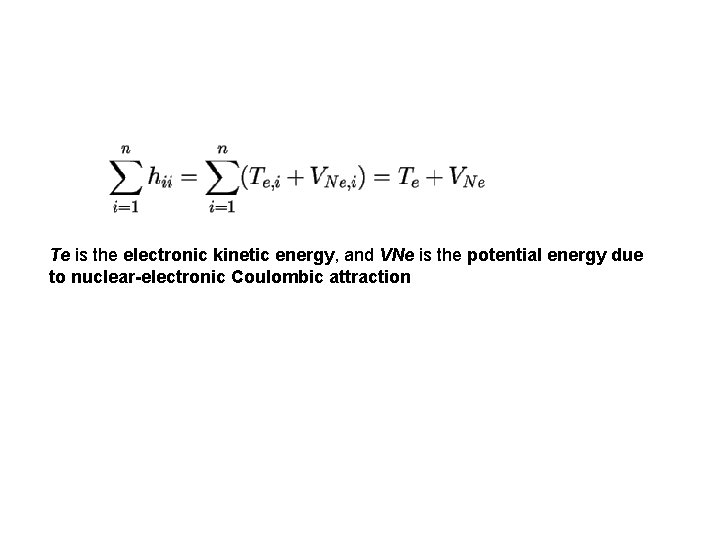
first term in this sum is an integral over a constant, since the nuclear-nuclear repulsion energy does not depend on the electronic coordinates VNN is the potential energy due to nuclear-nuclear Coulombic repulsion. which can be re-written as sums of integrals, leading to:

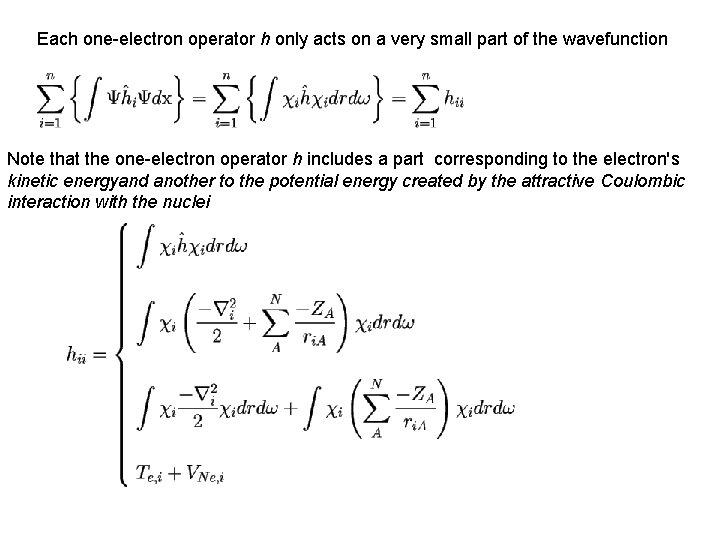
Each one-electron operator h only acts on a very small part of the wavefunction Note that the one-electron operator h includes a part corresponding to the electron's kinetic energyand another to the potential energy created by the attractive Coulombic interaction with the nuclei

Te is the electronic kinetic energy, and VNe is the potential energy due to nuclear-electronic Coulombic attraction

The second set of integrals, the two-electron terms The first and fourth, and second and third

2(r) is the probability of finding an electron at a given point in space this integral is often called the Coulomb Integral, written in shorthand as Jij. Because 1/r is always positive, this term contributes a positive energy Exchange Integral, and written as Kij, Exchange Integral comes from the fact that the two electrons exchange their positions from the left to the right of the integral. This suggests, correctly, that it has something to do with the Pauli principle.

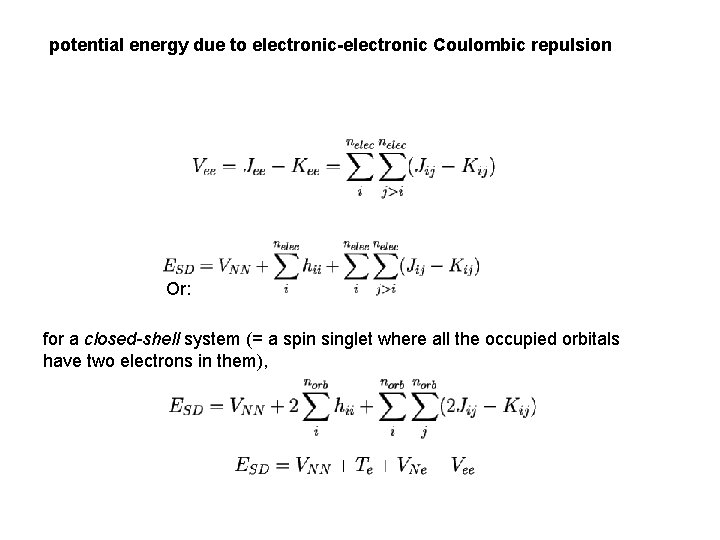
potential energy due to electronic-electronic Coulombic repulsion Or: for a closed-shell system (= a spin singlet where all the occupied orbitals have two electrons in them),

The Hartree-Fock-Roothaan Method




 Variational methods
Variational methods Hamiltonian operator
Hamiltonian operator Variational shape approximation
Variational shape approximation An introduction to variational methods for graphical models
An introduction to variational methods for graphical models Variational message passing
Variational message passing Variational approach in fem
Variational approach in fem D'alembert
D'alembert Variational calculus
Variational calculus Variational knowledge graph reasoning
Variational knowledge graph reasoning Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Objectives of symposium
Objectives of symposium Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Slidetodoc
Slidetodoc Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói