Uncertainty Principle Uncertainly Principle 1 Uncertainty Principle Summary







- Slides: 23

Uncertainty Principle Uncertainly Principle 1

Uncertainty Principle Summary of Bohr’s Model (1913) Electrons are in different orbits at fixed distances from nucleus. Electrons that leave one orbit must move to another orbit. Electrons only change orbits if specific amounts (quanta) of extra energy from the outside world are involved. Electrons that receive enough extra energy from the outside world can leave the atom they are in. Electrons that return to orbits they used to reside in give up the extra energy they acquired when they moved in the first place. Electronic energy given up when electrons move back into an original orbit often shows up as a specific color light. 2

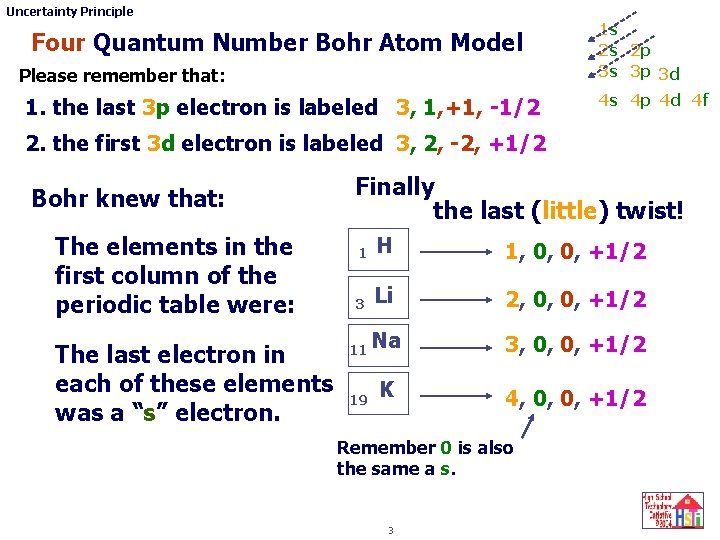
Uncertainty Principle Four Quantum Number Bohr Atom Model Please remember that: 1. the last 3 p electron is labeled 3, 1, +1, -1/2 1 s 2 s 2 p 3 s 3 p 3 d 4 s 4 p 4 d 4 f 2. the first 3 d electron is labeled 3, 2, -2, +1/2 Bohr knew that: The elements in the first column of the periodic table were: The last electron in each of these elements was a “s” electron. Finally the last (little) twist! 1 H 1, 0, 0, +1/2 3 Li 2, 0, 0, +1/2 11 Na 19 K 3, 0, 0, +1/2 4, 0, 0, +1/2 Remember 0 is also the same a s. 3

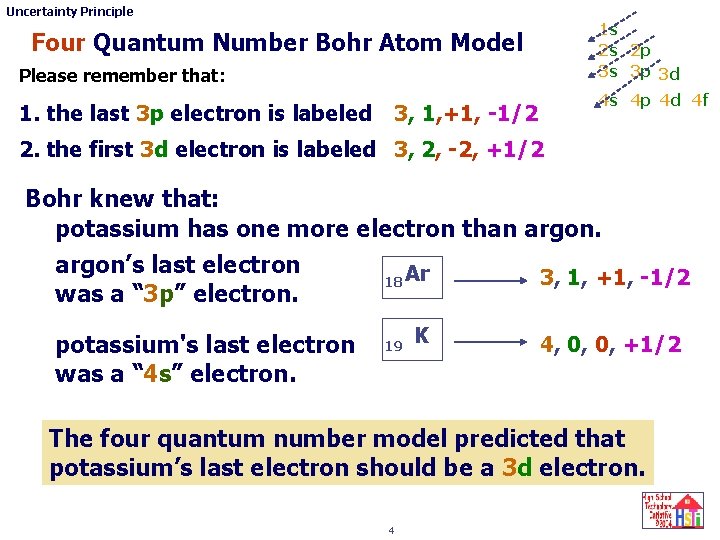
Uncertainty Principle 1 s 2 s 2 p 3 s 3 p 3 d Four Quantum Number Bohr Atom Model Please remember that: 1. the last 3 p electron is labeled 4 s 4 p 4 d 4 f 3, 1, +1, -1/2 2. the first 3 d electron is labeled 3, 2, -2, +1/2 Bohr knew that: potassium has one more electron than argon’s last electron 3, 1, +1, -1/2 18 Ar was a “ 3 p” electron. potassium's last electron was a “ 4 s” electron. 19 K 4, 0, 0, +1/2 The four quantum number model predicted that potassium’s last electron should be a 3 d electron. 4

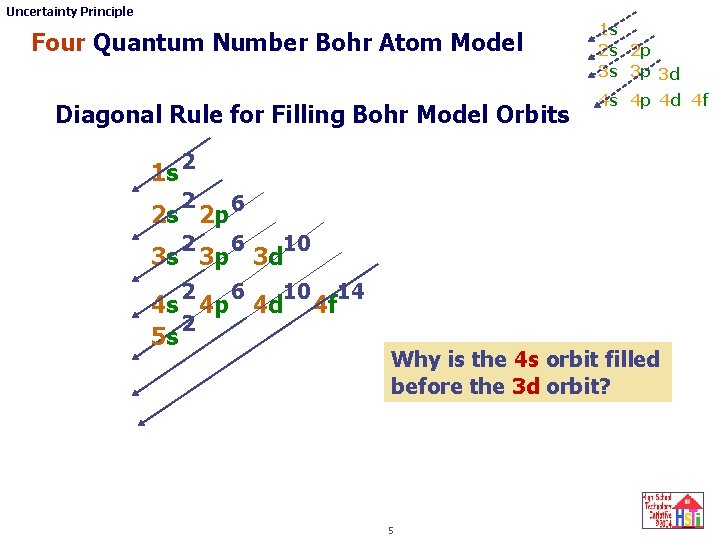
Uncertainty Principle Four Quantum Number Bohr Atom Model Diagonal Rule for Filling Bohr Model Orbits 1 s 2 s 2 p 3 s 3 p 3 d 4 s 4 p 4 d 4 f 1 s 2 2 2 s 2 p 6 2 6 10 3 s 3 p 3 d 2 6 10 14 4 s 4 p 4 d 4 f 2 5 s Why is the 4 s orbit filled before the 3 d orbit? 5

Uncertainty Principle Why is the 4 s orbit filled before the 3 d orbit? 1 s 2 s 2 p 3 s 3 p 3 d 4 s 4 p 4 d 4 f Electrons within an atom can be completely identified as unique electrons with the aid of 4 quantum numbers. These 4 quantum numbers are called: n the principal quantum number the angular momentum (azimuthal) quantum number m the magnetic quantum number s the electron spin quantum number l No two electrons in the same atom can have the same value for all four of these quantum numbers. 6

Uncertainty Principle Why is the 4 s orbit filled before the 3 d orbit? 1 s 2 s 2 p 3 s 3 p 3 d 4 s 4 p 4 d 4 f Electrons returning to their “ground” state can emit light with a unique frequency (energy). Atoms are filled with electrons from the orbit closest to the nucleus to the orbit furthest from the nucleus. The diagonal fill rule predicts the way electrons fill orbits. The Bohr model does not explain why the 4 s orbit is filled before the 3 d orbit. A revised model is needed to provide this explanation. 7

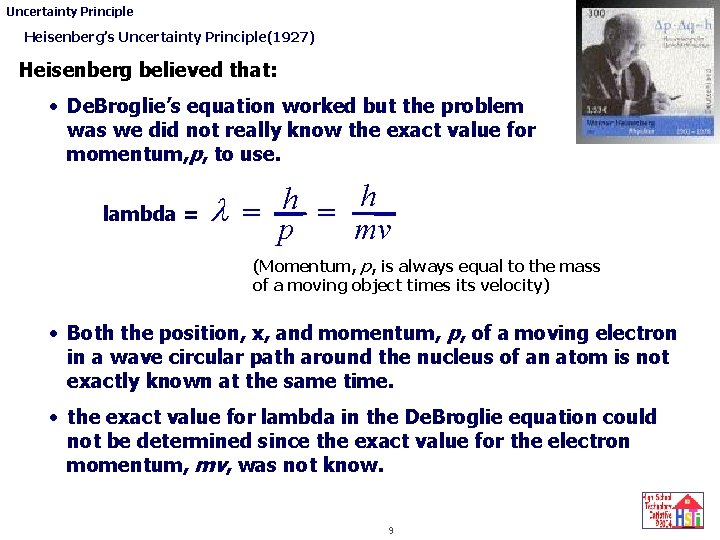
Uncertainty Principle Heisenberg’s Uncertainty Principle(1927) Heisenberg understood and agreed with: Planck’s ideas (1900) Einstein's ideas (1905) De. Broglie’s ideas (1924) Schrodinger’s ideas (1926) But he wondered why the Schrodinger wave equation did not work very well when there was more than one electron in the atom. 8

Uncertainty Principle Heisenberg’s Uncertainty Principle(1927) Heisenberg believed that: • De. Broglie’s equation worked but the problem was we did not really know the exact value for momentum, p, to use. lambda = h h l = = p mv (Momentum, p, is always equal to the mass of a moving object times its velocity) • Both the position, x, and momentum, p, of a moving electron in a wave circular path around the nucleus of an atom is not exactly known at the same time. • the exact value for lambda in the De. Broglie equation could not be determined since the exact value for the electron momentum, mv, was not know. 9

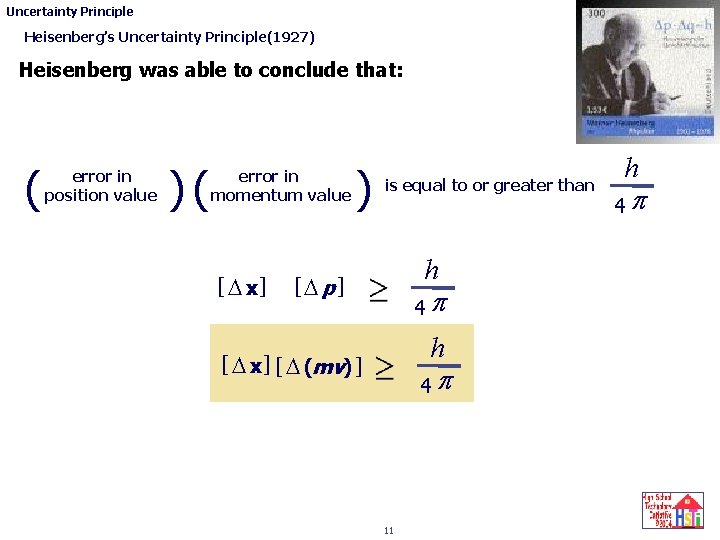
Uncertainty Principle Heisenberg’s Uncertainty Principle(1927) Heisenberg was able to conclude that: • Schrodinger’s wave equation would not describe electron movement around the nucleus very well since the electron’s wavelength would never be known with exact certainty. • Although Heisenberg could not work out what the exact uncertainty in the momentum value of a moving electron was, he was able to determine what the smallest value of the product of the distance uncertainty, [ D x ] , and the momentum uncertainty would be [ D mv ]. ( uncertainty in position value )( uncertainty in momentum value ) is equal to or greater than 10 h 4 p

Uncertainty Principle Heisenberg’s Uncertainty Principle(1927) Heisenberg was able to conclude that: ( uncertainty error in in position value )( uncertainty error in in momentum value [ D x] ) is equal to or greater than h [D p] 4 p h [ D x ] [ D (mv) ] 4 p 11 h 4 p

Uncertainty Principle The Post Bohr Atom By 1930, a new picture of the electron arrangement and position had developed and this theory is the one still used today. • An electron’s position is not possible to determine. • It is only possible to determine the probability of where an electron might be at any given instant of time. • The solution to the Wave Equation for a range of uncertain x values determines the 3 -D shapes about the atom’s nucleus within which electrons will most likely be located. 12

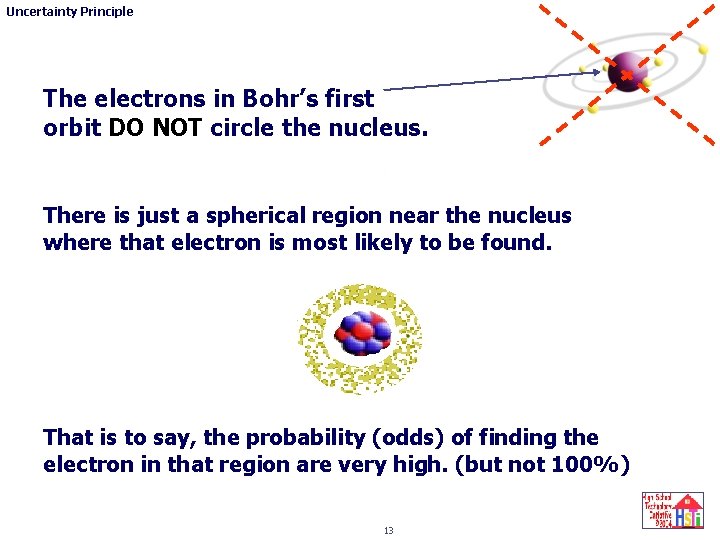
Uncertainty Principle The electrons in Bohr’s first orbit DO NOT circle the nucleus. There is just a spherical region near the nucleus where that electron is most likely to be found. That is to say, the probability (odds) of finding the electron in that region are very high. (but not 100%) 13

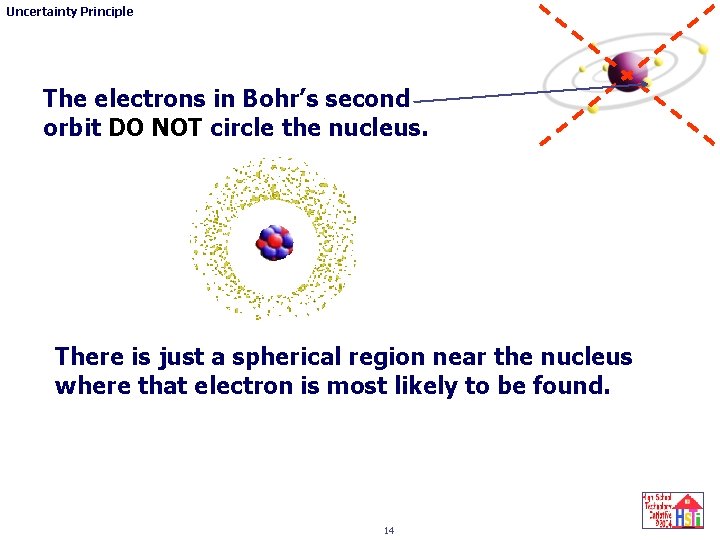
Uncertainty Principle The electrons in Bohr’s second orbit DO NOT circle the nucleus. There is just a spherical region near the nucleus where that electron is most likely to be found. 14

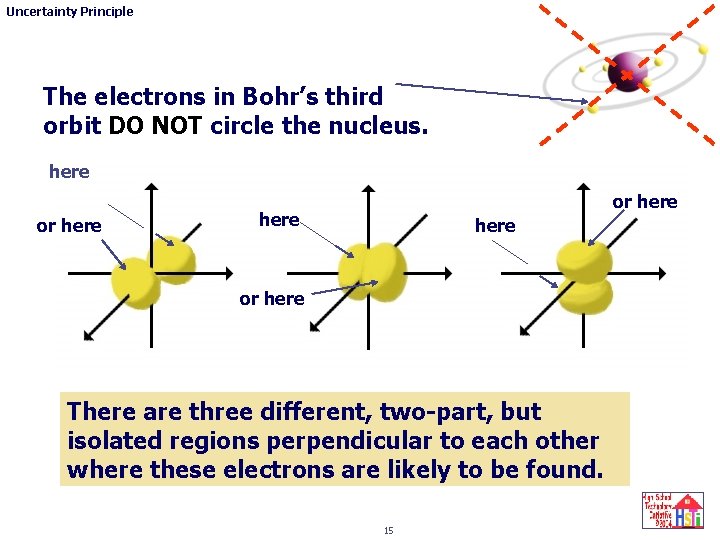
Uncertainty Principle The electrons in Bohr’s third orbit DO NOT circle the nucleus. here or here There are three different, two-part, but isolated regions perpendicular to each other where these electrons are likely to be found. 15

Uncertainty Principle An electron can also be located further from the nucleus in one of 5 d regions. There are five different multiple but isolated regions perpendicular to each other where that electron is likely to be found. 16

Uncertainty Principle The Post Bohr Atom This new picture of the electron arrangement and position about the nucleus answers many of Bohr’s questions. One of the first questions that was answered was why the DIAGONAL RULE for filling orbitals with electrons works. 17

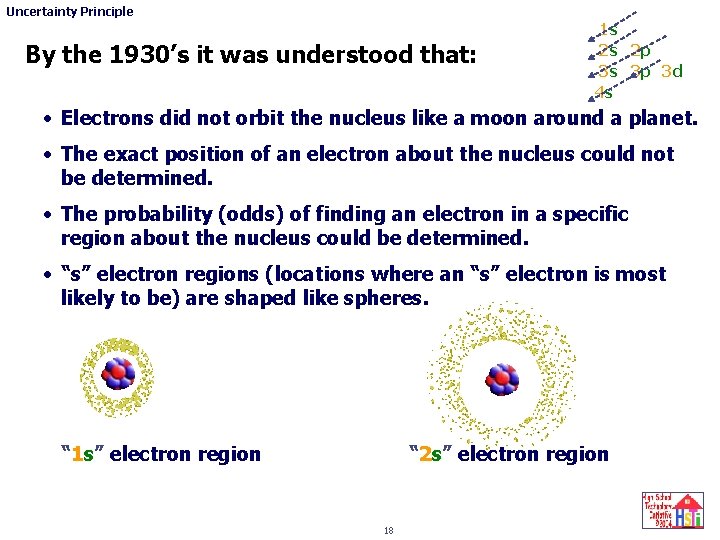
Uncertainty Principle By the 1930’s it was understood that: 1 s 2 s 2 p 3 s 3 p 3 d 4 s • Electrons did not orbit the nucleus like a moon around a planet. • The exact position of an electron about the nucleus could not be determined. • The probability (odds) of finding an electron in a specific region about the nucleus could be determined. • “s” electron regions (locations where an “s” electron is most likely to be) are shaped like spheres. “ 1 s” electron region “ 2 s” electron region 18

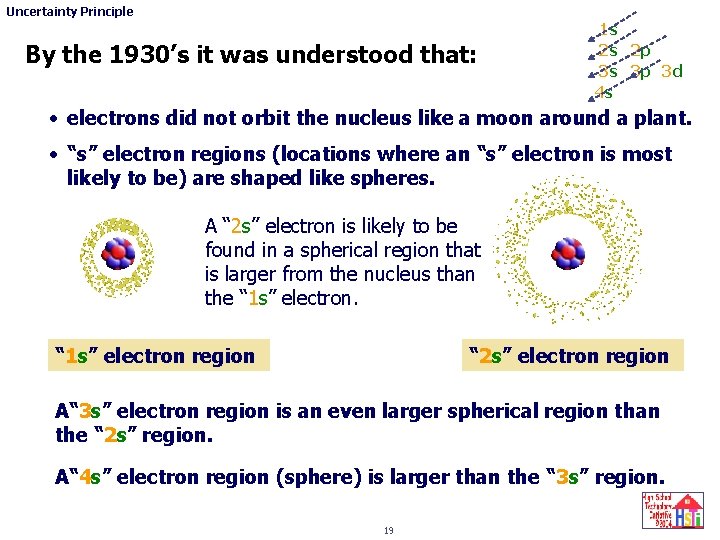
Uncertainty Principle By the 1930’s it was understood that: 1 s 2 s 2 p 3 s 3 p 3 d 4 s • electrons did not orbit the nucleus like a moon around a plant. • “s” electron regions (locations where an “s” electron is most likely to be) are shaped like spheres. A “ 2 s” electron is likely to be found in a spherical region that is larger from the nucleus than the “ 1 s” electron region “ 2 s” electron region A“ 3 s” electron region is an even larger spherical region than the “ 2 s” region. A“ 4 s” electron region (sphere) is larger than the “ 3 s” region. 19

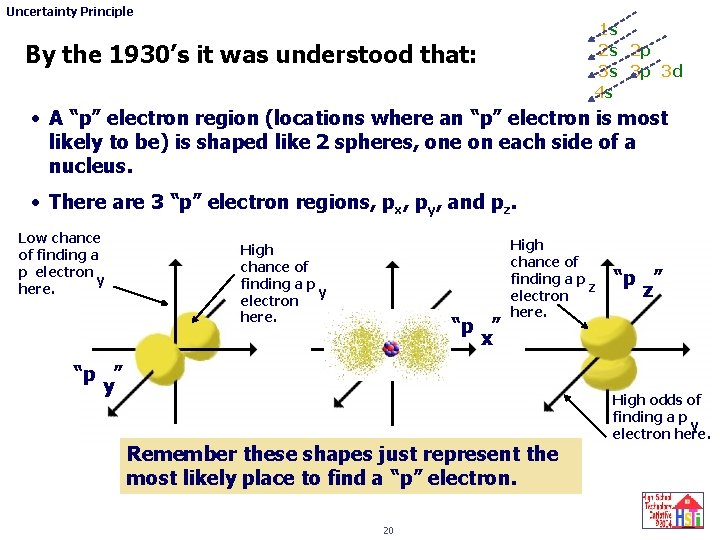
Uncertainty Principle 1 s 2 s 2 p 3 s 3 p 3 d 4 s By the 1930’s it was understood that: • A “p” electron region (locations where an “p” electron is most likely to be) is shaped like 2 spheres, one on each side of a nucleus. • There are 3 “p” electron regions, px, py, and pz. Low chance of finding a p electron y here. High chance of finding a p y electron here. “p ” x High chance of finding a p z electron here. “p ” y Remember these shapes just represent the most likely place to find a “p” electron. 20 “p ” z High odds of finding a p y electron here.

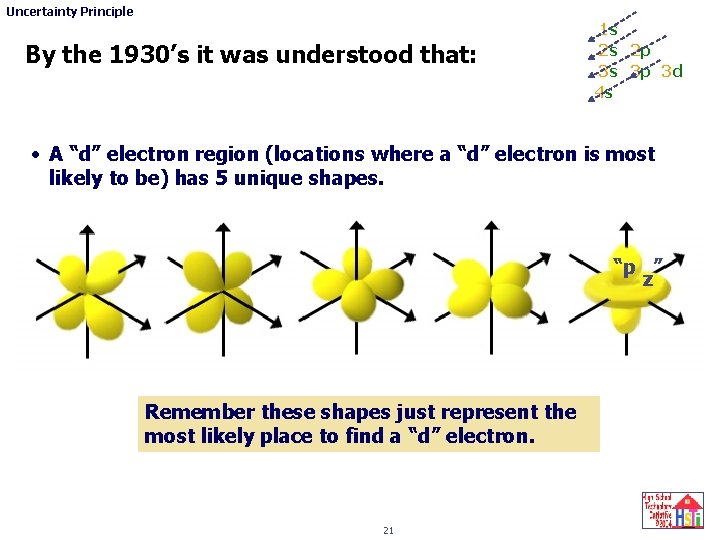
Uncertainty Principle By the 1930’s it was understood that: 1 s 2 s 2 p 3 s 3 p 3 d 4 s • A “d” electron region (locations where a “d” electron is most likely to be) has 5 unique shapes. “p ” z Remember these shapes just represent the most likely place to find a “d” electron. 21

Uncertainty Principle By the 1930’s it was understood that: 1 s 2 s 2 p 3 s 3 p 3 d 4 s 4 p • The 4 s region has a very large radius out from the nucleus and represents a very large spherical space. • There is a high probability that an electron will go into the 4 s region instead of the 3 d region because the 4 s region overlaps some of the 3 d region space. Thus, the diagonal rule works, that is to say, the 4 s region will fill up before an electron enters the 3 d region. What orbit starts to fill after the 3 d orbit has been filled? 22

Uncertainty Principle 23