The Development of Atomic Theory Earliest Ideas of

















- Slides: 22

The Development of Atomic Theory

Earliest Ideas of Atoms • • Democritus- 4 B. C. Believed that matter was made of tiny indivisible units. He coined the term atom to describe this particle, which means “indivisible” in Greek. Conclusions were not supported by experimental evidence.

Foundations of Atomic Theory • • • 1700 s- Several scientists were exploring with elements experimentally General beliefs of the time: 1) Elements are substances that cannot be broken down by ordinary chemical means. 2) Elements combine to form compounds. However, they were not sure if elements always combine in the same ratios.

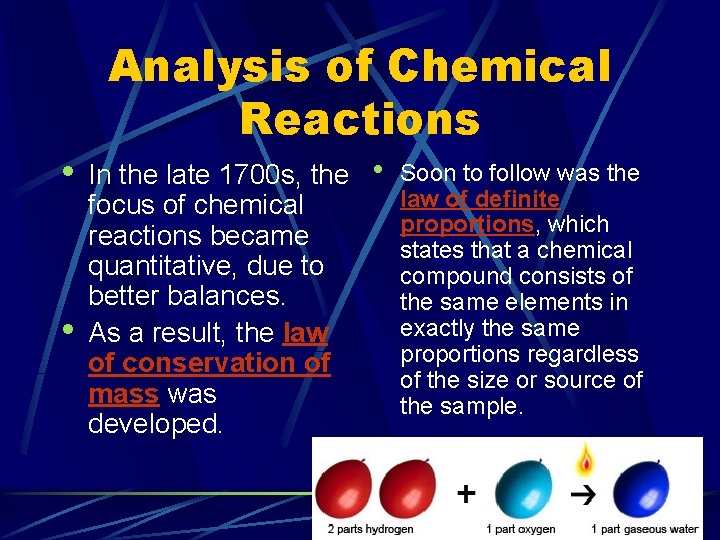
Analysis of Chemical Reactions • • In the late 1700 s, the focus of chemical reactions became quantitative, due to better balances. As a result, the law of conservation of mass was developed. • Soon to follow was the law of definite proportions, which states that a chemical compound consists of the same elements in exactly the same proportions regardless of the size or source of the sample.

Analysis of Chemical Reactions • • • As you might have thought, there are often more than one compound that can be created given two elements. ie: CO and CO 2 So, the law of multiple proportions states: If two or more different compounds are composed of the same two elements, then the ratio of the masses of the second element combined with a certain mass of the first is always a ratio of small numbers.

Dalton’s Atomic Theory • John Dalton, an English schoolteacher, • • developed his theory in 1808. This theory was an explanation for the previous three laws. His theory can be summed up in five statements:

Dalton’s Theory • • • 1) All matter is composed of atoms. 2) Atoms of a given element are identical in size, mass and other properties. 3) Atoms cannot be subdivided, created or destroyed. 4) Atoms of different elements combine in simple whole number ratios to form compounds. 5) In chemical reactions, atoms are combined, separated or rearranged.

Modern Atomic Theory • Now, we know that some of Dalton’s • • theory is incorrect. Specifically, we know that atoms can be divided into smaller particles. We also know that atoms of a given element have different masses. However, Dalton contributed greatly to our knowledge of atomic structure.

Structure of the Atom • Atom- smallest particle of an element • • that retains the chemical properties of that element. Consists of a nucleus, consisting of protons and neutrons. Surrounding the nucleus is an electron cloud.

How do we know about subatomic particles? ? • In a phrase…experimental observations. • It began with the discovery of the electron. • This discovery came out of an • experiment with a cathode ray tube. Cathode ray tubes send an electric current through a gas at low pressure.

Electron Discovery • • • Experiments were done to prove the existence of a ray of particles. 1) A paddle wheel placed on rails between the cathode and anode moved. 2) The ray was deflected by a magnetic field, just like a wire carrying a negative current. (proving a negative charge of the particles)

Thomson’s Experiments • • • JJ Thomson- 1897 Used cathode ray tubes to investigate electrons. He was able to measure the ratio of the charge of the cathode ray particles to their mass. He discovered that the ratio was always the same, regardless of the metal used to make the cathode or the gas in the tube. Essentially he discovered electrons!

Thomson’s Plum Pudding Model • Since atoms are indeed neutral, he suggested that positive and negative charges were spread throughout the atom. (just like the plums in plum pudding)

Millikan’s Oil Drop Experiment • • • Robert Millikan determined that the mass of an electron is about 1/2000 that of the simplest type of hydrogen atom. To do this, he used atomized oil drops that fell between two parallel electrode plates. Knowing the charge of the electric field, he could determine the charge on an oil droplet.

Discovery of the Atomic Nucleus • • • 1911 - Gold foil experiment conducted by Ernest Rutherford and associates, Hans Geiger and Ernest Marsden Gold foil was bombarded with alpha particles with a radioactive source. It was assumed that the charges were uniformly distributed throughout the atom, so they thought the particles would pass right through.

Gold Foil Experiment • For the majority of particles, they were • • correct… the particles passed right through. However, some were widely deflected, and 1 in 8000 bounced back! Rutherford exclaimed, “It was as if you had fired a 15 inch artillery shell at a piece of tissue and it came back and hit you. ”


Conclusions • After two long years, an explanation • • was finally reached. The rebounded atoms must have collided with a powerful force in the atom. The force must be caused by a densely packed region in the atom… he called it the nucleus.

The missing piece • Neutrons were not discovered until 1932 by • • James Chadwick. He conducted an experiment similar to Rutherford, but he used boron instead of gold foil. Chadwick determine that the neutral particle that was coming out as radiation was not light, or gamma radiation but a neutron. He went on to determine its mass.

Bohr Model of the Atom • Niels Bohr thought that electrons moved in • • specific paths around the nucleus, like planets orbit the sun. However, his model only worked for the element hydrogen. Why do you think this is the case? When additional electrons were added, the repulsion between them played a role in how electrons moved and where electrons are in the atom.

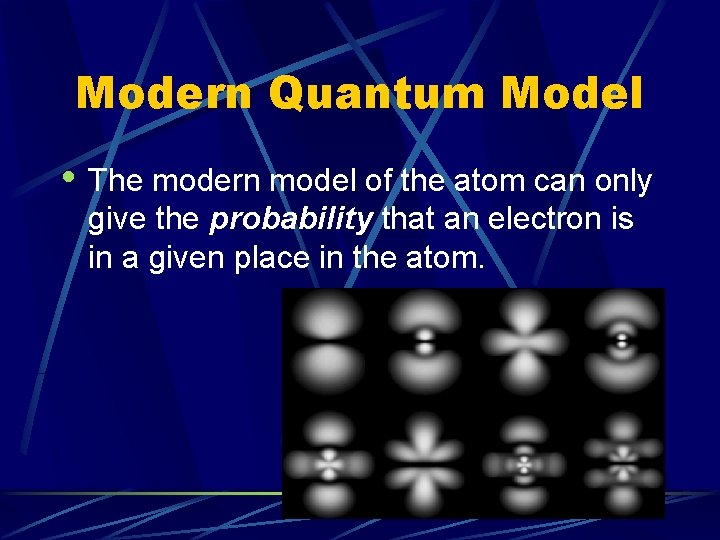
Modern Quantum Model • The modern model of the atom can only give the probability that an electron is in a given place in the atom.

Size of an atom • The radius of an atom is the distance • • from the center of the nucleus to the outer portion of its electron cloud. To measure this distance, picometers (pm) are used. 1 pm = 10 -12 m = 10 -10 cm Atomic radii range from 40 -270 pm. The radius of a nucleus is 0. 001 pm.
 Relative atomic mass of beryllium
Relative atomic mass of beryllium Trends on the periodic table
Trends on the periodic table Atomic radius vs ionic radius
Atomic radius vs ionic radius Isotope abundance formula
Isotope abundance formula Difference between atomic mass and atomic number
Difference between atomic mass and atomic number Atomic number vs atomic radius
Atomic number vs atomic radius Define urban hierarchy
Define urban hierarchy Earliest urban hearths
Earliest urban hearths The are our earliest literature
The are our earliest literature The earliest bridges consisted mainly of
The earliest bridges consisted mainly of Prometheus background
Prometheus background Earliest chordates
Earliest chordates Earliest urban hearths
Earliest urban hearths Earliest start time formula
Earliest start time formula Cradle of genius rizal
Cradle of genius rizal Pericles and the bull
Pericles and the bull Earliest form of tennis was called
Earliest form of tennis was called Indian people called gymnastics as naked art
Indian people called gymnastics as naked art The earliest fossils date back to about when?
The earliest fossils date back to about when? Earliest earth
Earliest earth What civilization did the himalayas separate china from
What civilization did the himalayas separate china from Advantages of interactional model of communication
Advantages of interactional model of communication Oldest genre
Oldest genre