Structural Analysis Apurva Mehta Physics of Diffraction Xray

- Slides: 40

Structural Analysis Apurva Mehta

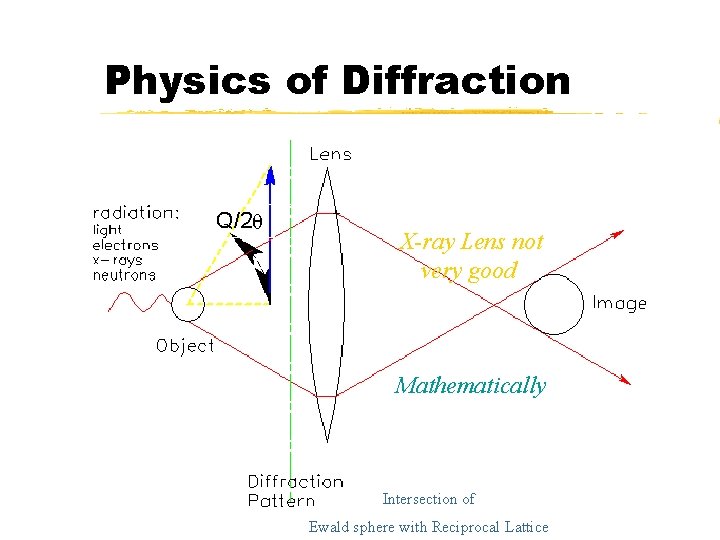
Physics of Diffraction X-ray Lens not very good Mathematically Intersection of Ewald sphere with Reciprocal Lattice

outline v. Information in a Diffraction pattern v. Structure Solution v. Refinement Methods v. Pointers for Refinement quality data

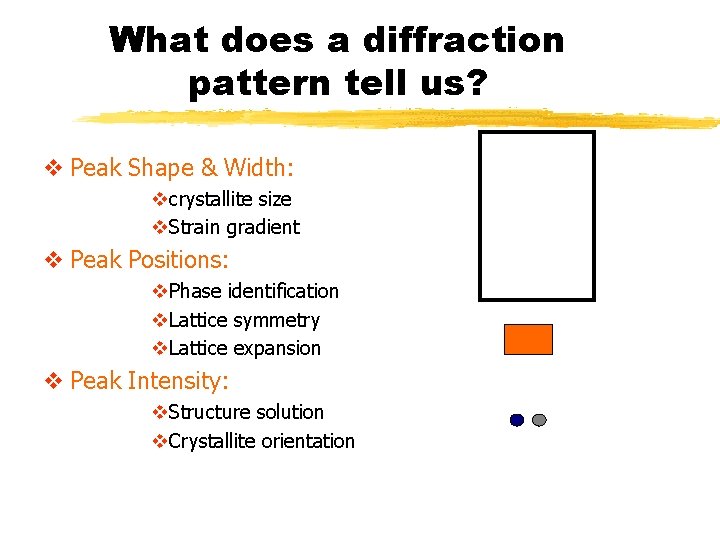
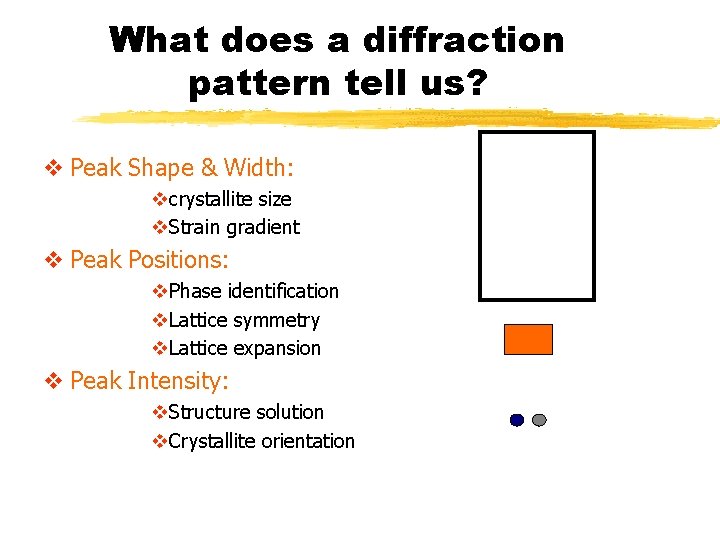
What does a diffraction pattern tell us? v Peak Shape & Width: vcrystallite size v. Strain gradient v Peak Positions: v. Phase identification v. Lattice symmetry v. Lattice expansion v Peak Intensity: v. Structure solution v. Crystallite orientation

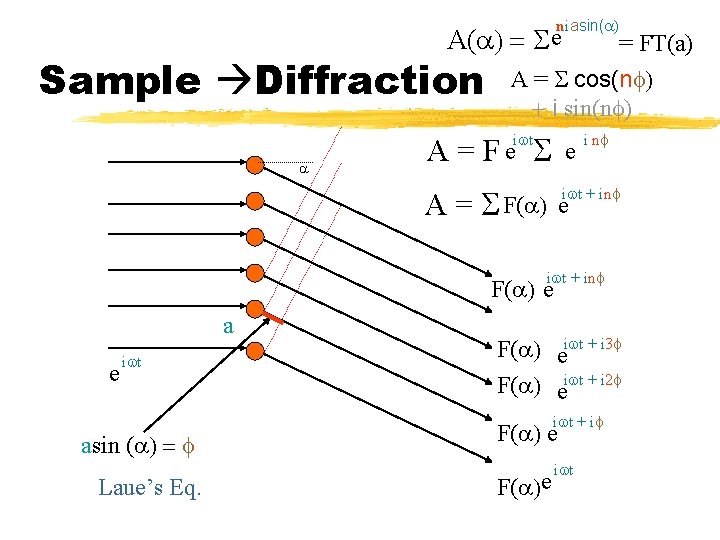
niasin(a) A(a) = S e Sample Diffraction a A= = FT(a) A = S cos(nf) + i sin(nf) S iwt Fe e A = S F(a) i nf iwt + inf e iwt + inf F(a) e a e iwt asin (a) = f Laue’s Eq. F(a) eiwt + i 3 f F(a) eiwt + i 2 f iwt + if F(a) e F(a)e iwt

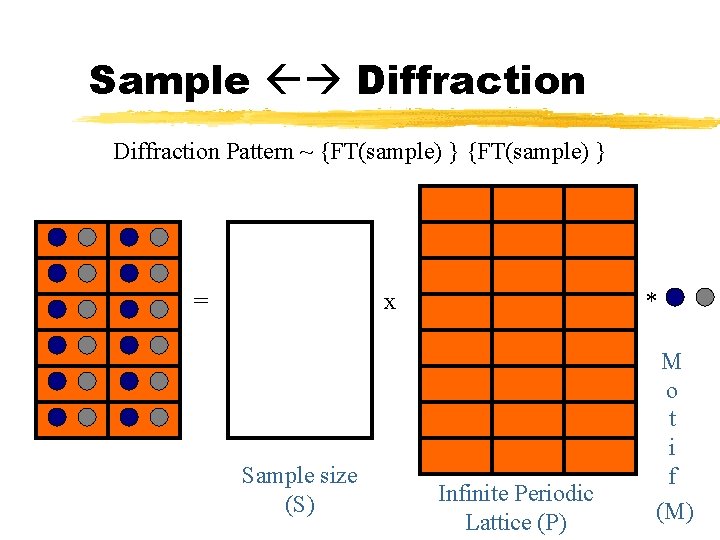
Sample Diffraction Pattern ~ {FT(sample) } = x Sample size (S) * Infinite Periodic Lattice (P) M o t i f (M)

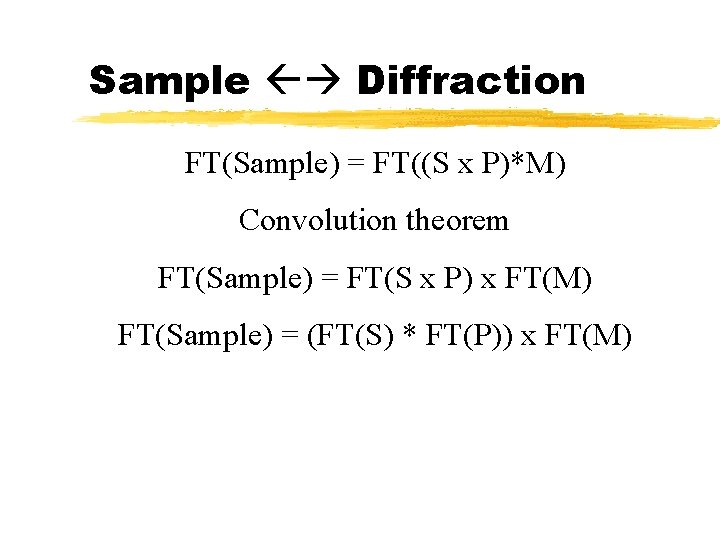
Sample Diffraction FT(Sample) = FT((S x P)*M) Convolution theorem FT(Sample) = FT(S x P) x FT(M) FT(Sample) = (FT(S) * FT(P)) x FT(M)

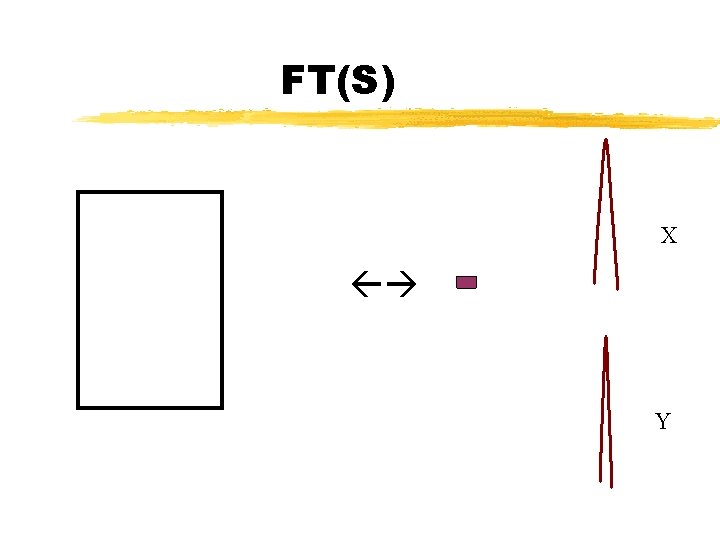
FT(S) X Y

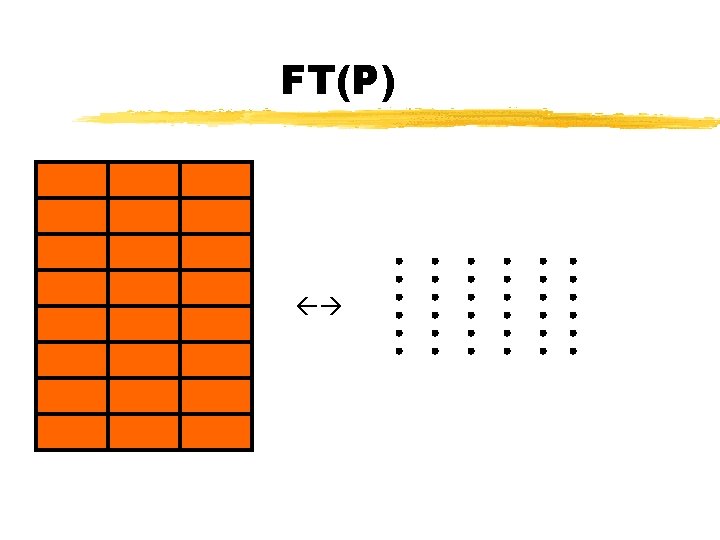
FT(P)

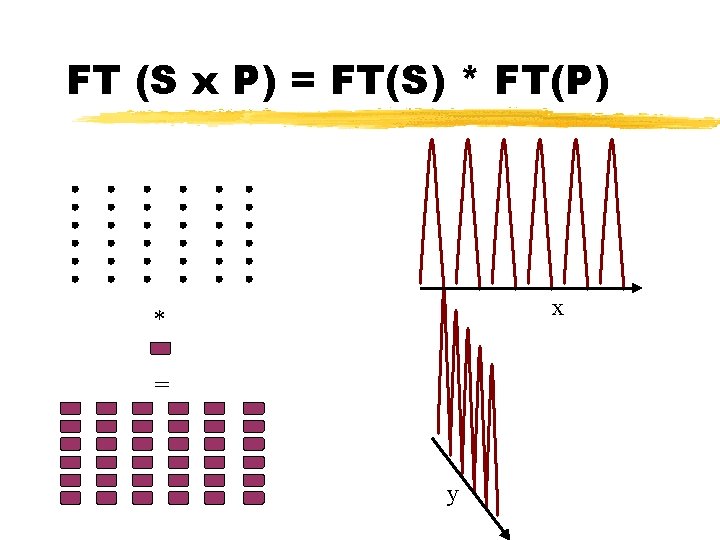
FT (S x P) = FT(S) * FT(P) x * = y

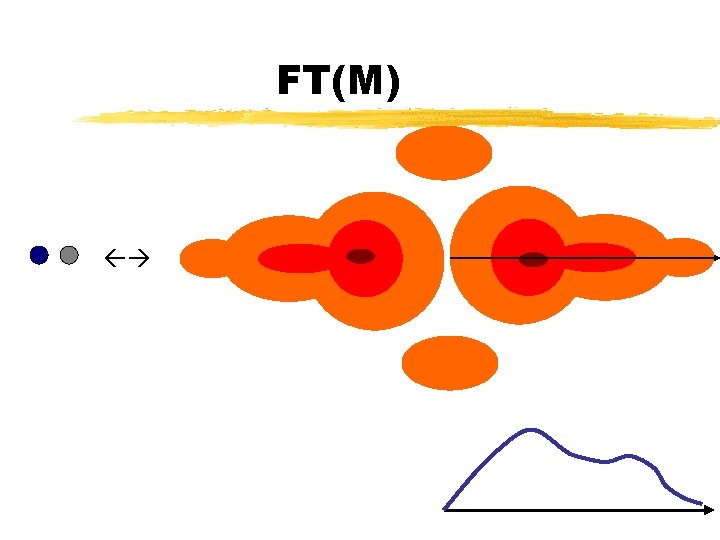
FT(M)

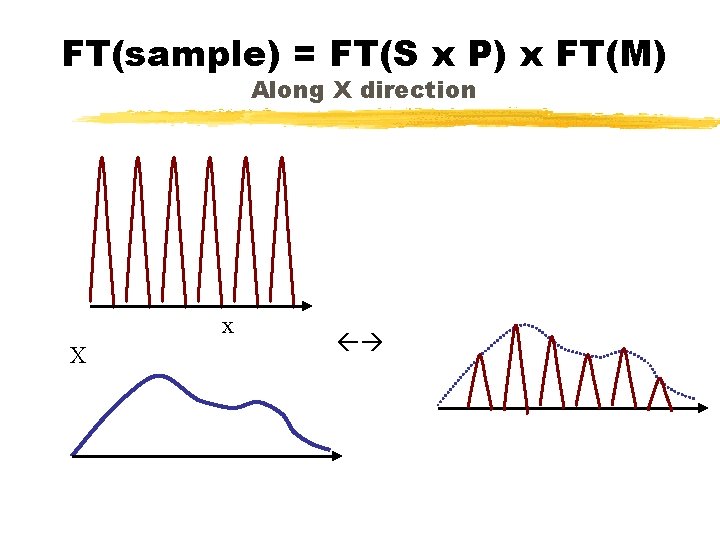
FT(sample) = FT(S x P) x FT(M) Along X direction x X

What does a diffraction pattern tell us? v Peak Shape & Width: vcrystallite size v. Strain gradient v Peak Positions: v. Phase identification v. Lattice symmetry v. Lattice expansion v Peak Intensity: v. Structure solution v. Crystallite orientation

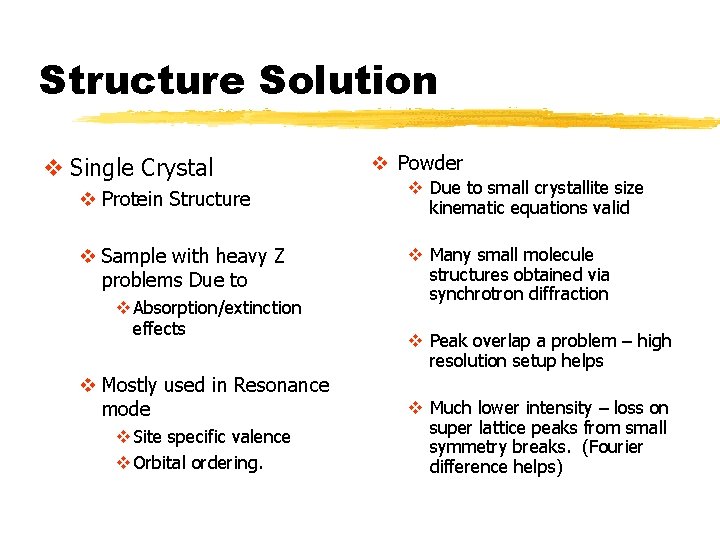
Structure Solution v Single Crystal v Powder v Protein Structure v Due to small crystallite size kinematic equations valid v Sample with heavy Z problems Due to v Many small molecule structures obtained via synchrotron diffraction v Absorption/extinction effects v Mostly used in Resonance mode v Site specific valence v Orbital ordering. v Peak overlap a problem – high resolution setup helps v Much lower intensity – loss on super lattice peaks from small symmetry breaks. (Fourier difference helps)

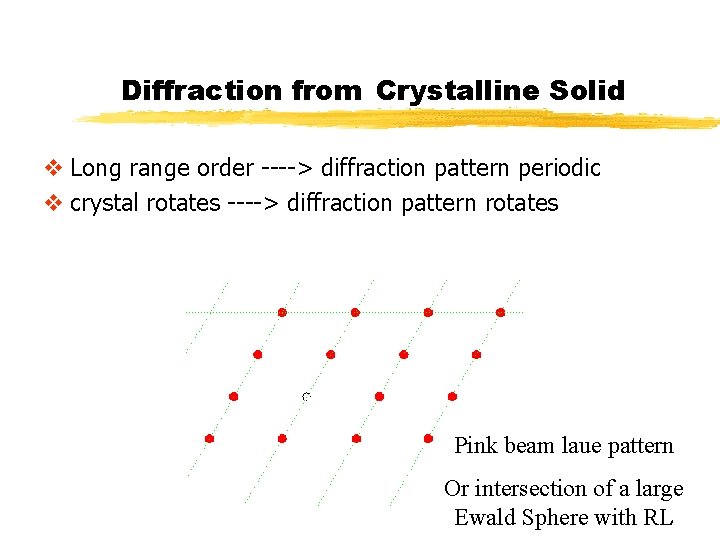
Diffraction from Crystalline Solid v Long range order ----> diffraction pattern periodic v crystal rotates ----> diffraction pattern rotates Pink beam laue pattern Or intersection of a large Ewald Sphere with RL

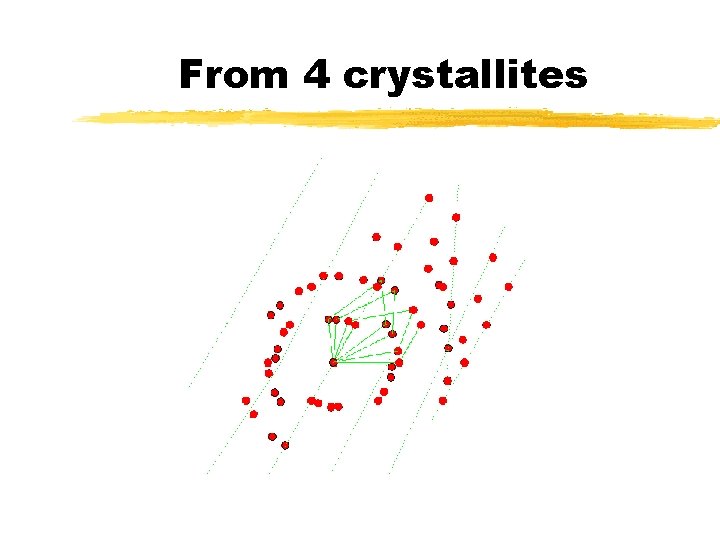
From 4 crystallites

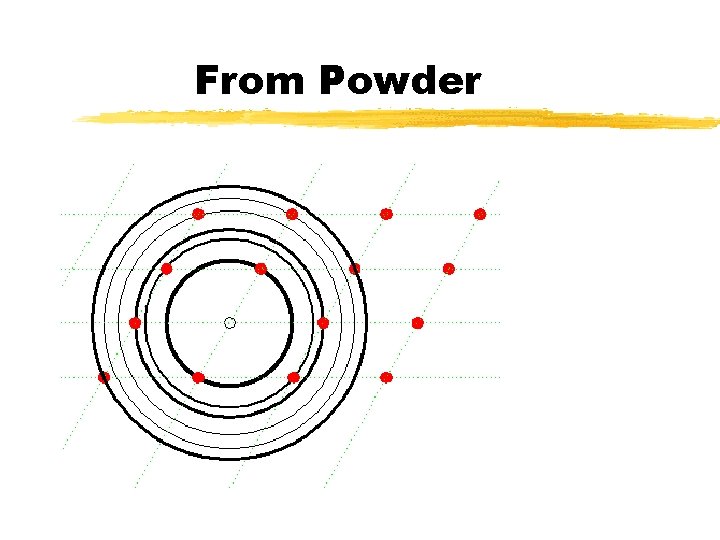
From Powder

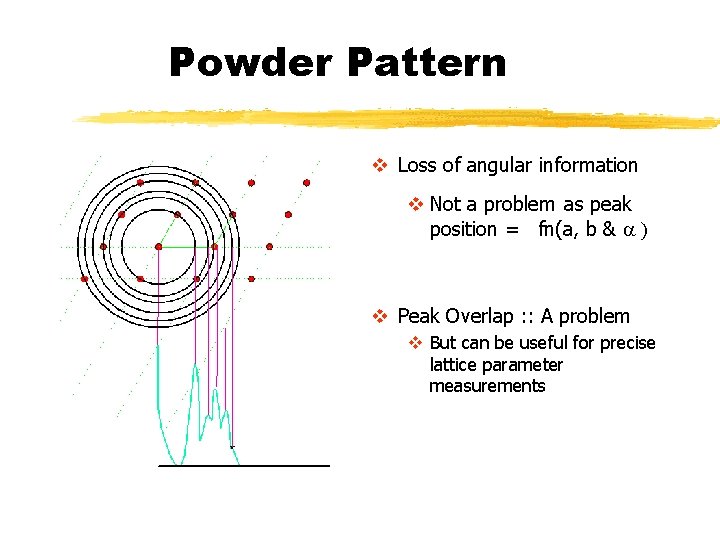
Powder Pattern v Loss of angular information v Not a problem as peak position = fn(a, b & a ) v Peak Overlap : : A problem v But can be useful for precise lattice parameter measurements

Peak Broadening v ~ (invers. ) “size” of the sample v. Crystallite size v. Domain size v. Strain & strain gradient v Diffractometer resolution should be better than Peak broadening But not much better.

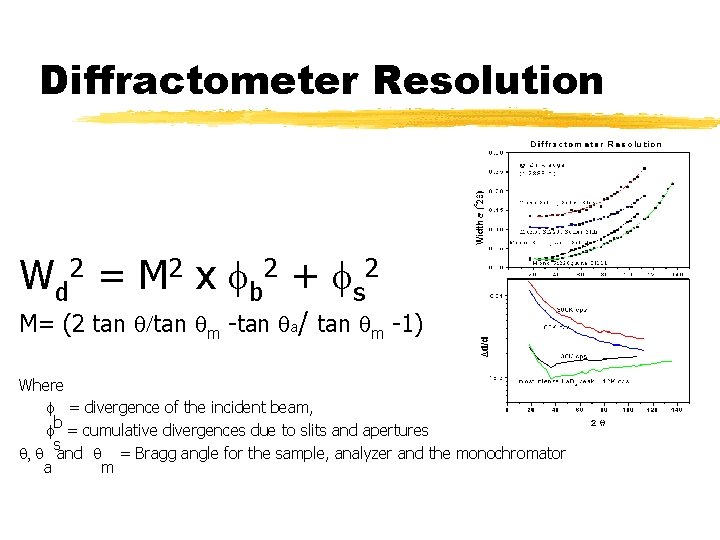
Diffractometer Resolution W d 2 = M 2 x f b 2 + f s 2 M= (2 tan q/tan qm -tan qa/ tan qm -1) Where f = divergence of the incident beam, b f = cumulative divergences due to slits and apertures s q, q and q = Bragg angle for the sample, analyzer and the monochromator a m

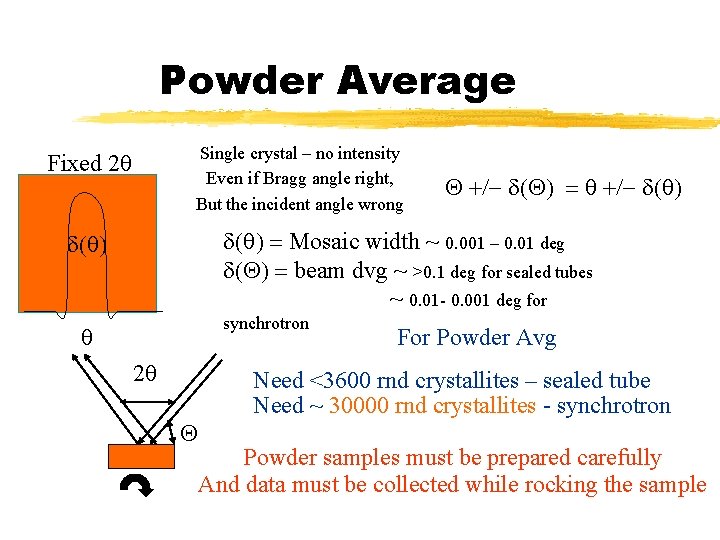
Powder Average Single crystal – no intensity Even if Bragg angle right, But the incident angle wrong Fixed 2 q Q +/- d(Q) = q +/- d(q) = Mosaic width ~ 0. 001 – 0. 01 deg d(Q) = beam dvg ~ >0. 1 deg for sealed tubes ~ 0. 01 - 0. 001 deg for d(q) synchrotron q 2 q Q For Powder Avg Need <3600 rnd crystallites – sealed tube Need ~ 30000 rnd crystallites - synchrotron Powder samples must be prepared carefully And data must be collected while rocking the sample

Physics of Diffraction No X-ray Lens Mathematically

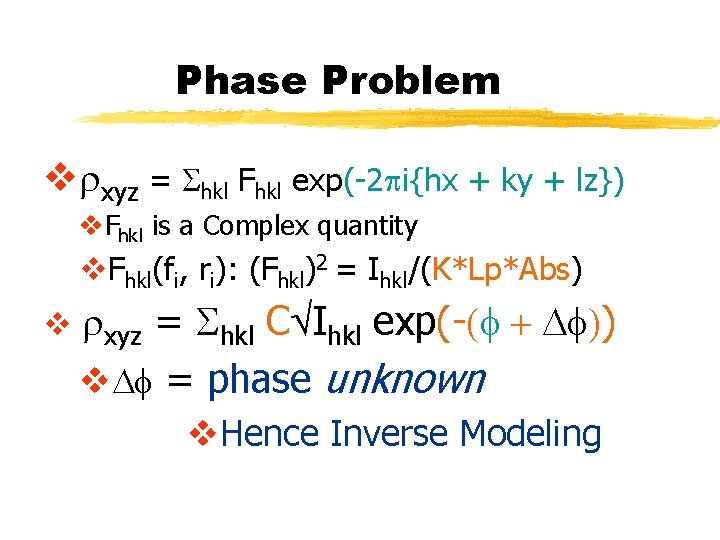
Phase Problem vrxyz = Shkl Fhkl exp(-2 pi{hx + ky + lz}) v. Fhkl is a Complex quantity v. Fhkl(fi, ri): (Fhkl)2 = Ihkl/(K*Lp*Abs) v rxyz = Shkl CÖIhkl exp(-(f + Df)) v. Df = phase unknown v. Hence Inverse Modeling

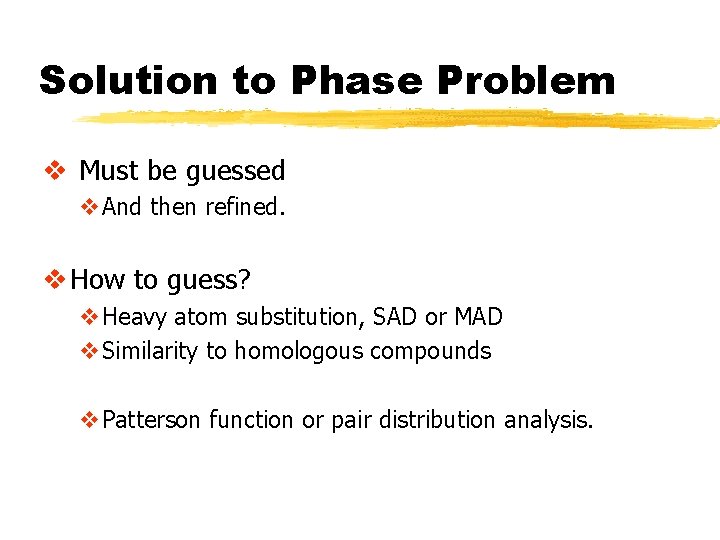
Solution to Phase Problem v Must be guessed v. And then refined. v How to guess? v. Heavy atom substitution, SAD or MAD v. Similarity to homologous compounds v. Patterson function or pair distribution analysis.

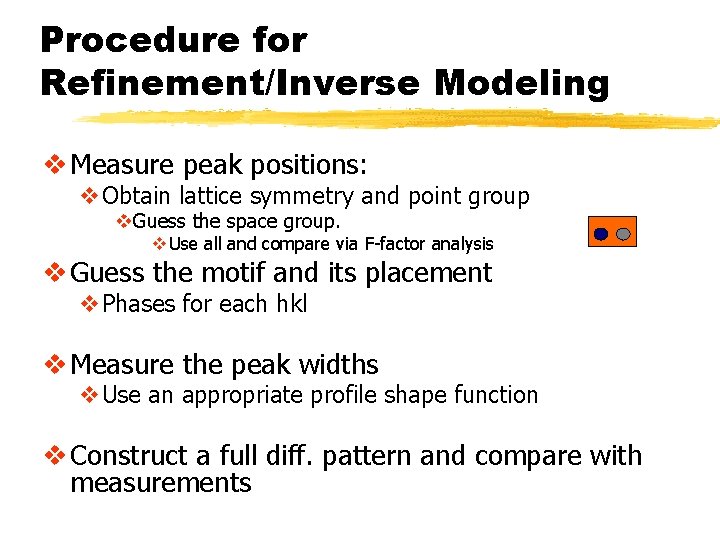
Procedure for Refinement/Inverse Modeling v Measure peak positions: v. Obtain lattice symmetry and point group v. Guess the space group. v Use all and compare via F-factor analysis v Guess the motif and its placement v. Phases for each hkl v Measure the peak widths v. Use an appropriate profile shape function v Construct a full diff. pattern and compare with measurements

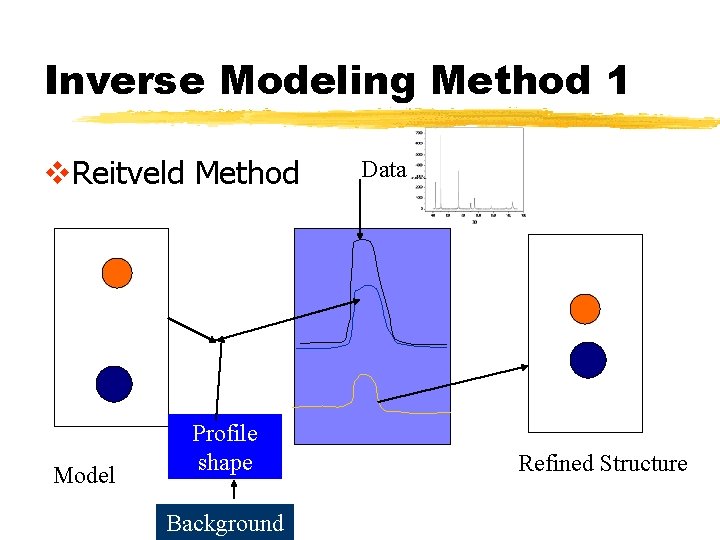
Inverse Modeling Method 1 v. Reitveld Method Model Profile shape Background Data Refined Structure

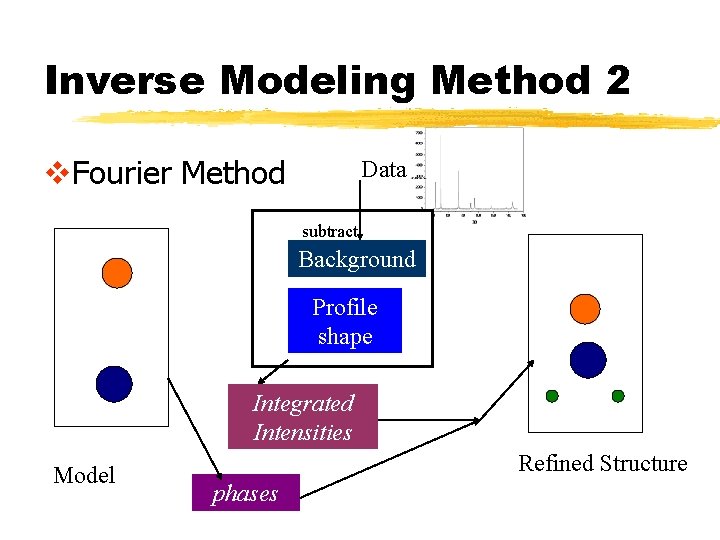
Inverse Modeling Method 2 v. Fourier Method Data subtract Background Profile shape Integrated Intensities Model Refined Structure phases

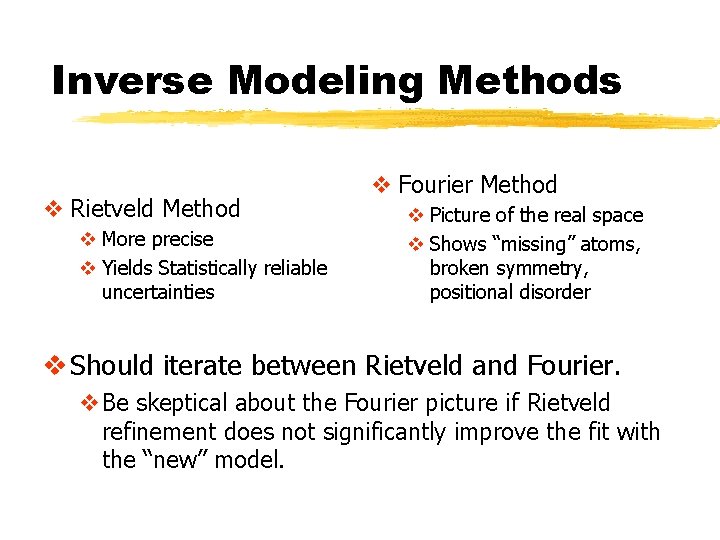
Inverse Modeling Methods v Rietveld Method v More precise v Yields Statistically reliable uncertainties v Fourier Method v Picture of the real space v Shows “missing” atoms, broken symmetry, positional disorder v Should iterate between Rietveld and Fourier. v. Be skeptical about the Fourier picture if Rietveld refinement does not significantly improve the fit with the “new” model.

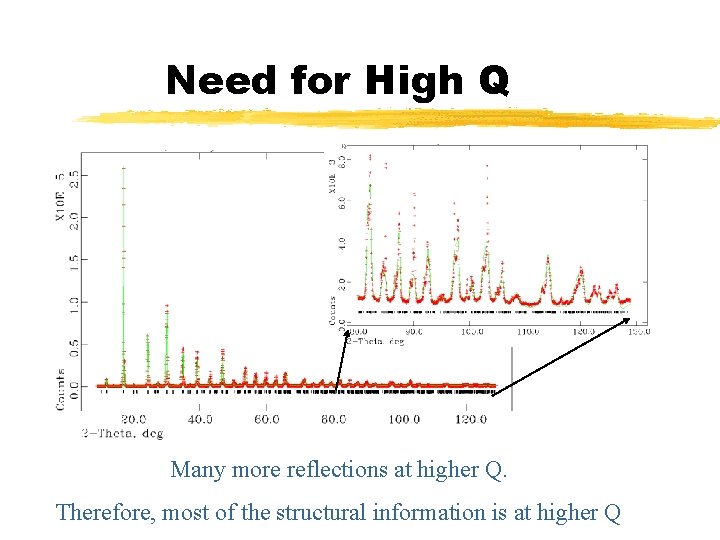
Need for High Q Many more reflections at higher Q. Therefore, most of the structural information is at higher Q

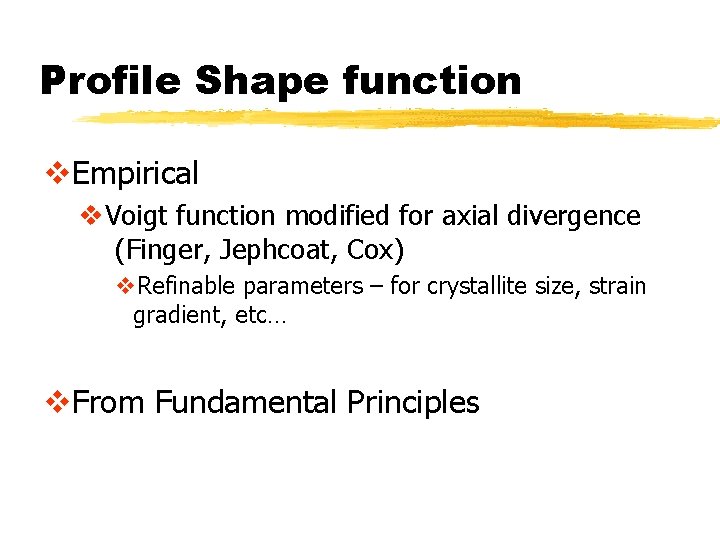
Profile Shape function v. Empirical v. Voigt function modified for axial divergence (Finger, Jephcoat, Cox) v. Refinable parameters – for crystallite size, strain gradient, etc… v. From Fundamental Principles

Collect data on Calibrant under the same conditions v. Obtain accurate wavelength and diffractometer misalignment parameters v. Obtain the initial values for the profile function (instrumental only parameters) v. Refine polarization factor v. Tells of other misalignment and problems

Selected list of Programs v. CCP 14 for a more complete list http: //www. ccp 14. ac. uk/mirror/want_to_do. html v. GSAS v. Fullprof v. DBW v. MAUD v. Topaz – not free - Bruker – fundamental approach

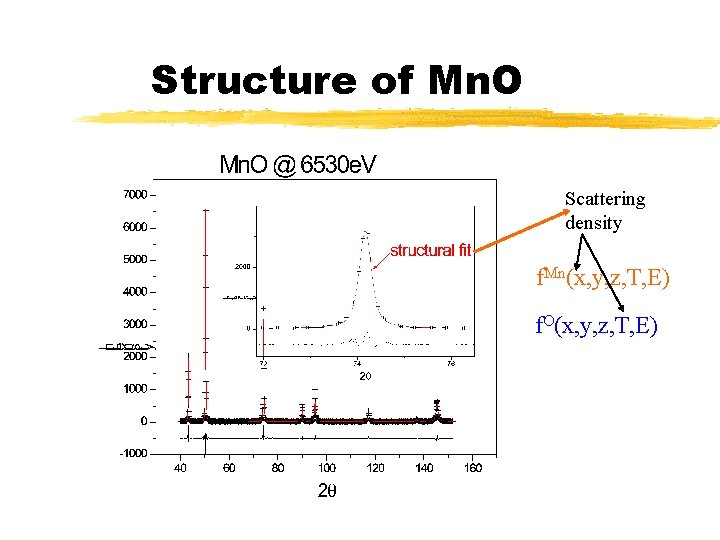
Structure of Mn. O Scattering density f. Mn(x, y, z, T, E) f. O(x, y, z, T, E)

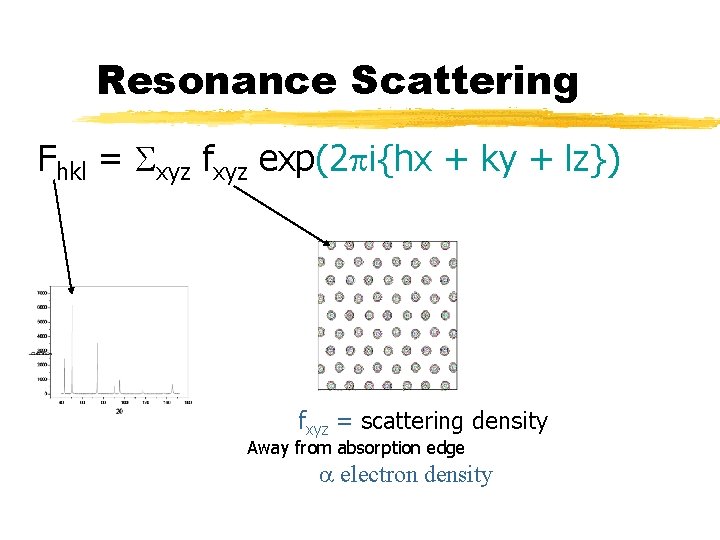
Resonance Scattering Fhkl = Sxyz fxyz exp(2 pi{hx + ky + lz}) fxyz = scattering density Away from absorption edge a electron density

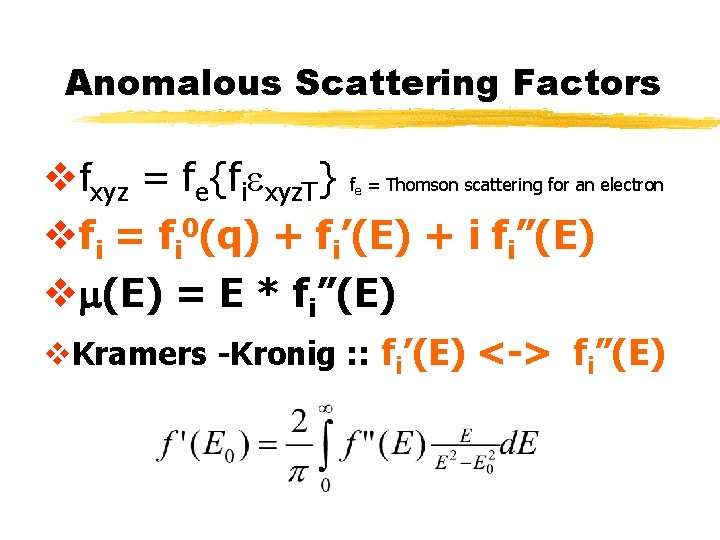
Anomalous Scattering Factors vfxyz = fe{fiexyz. T} f = Thomson scattering for an electron vfi = fi 0(q) + fi’(E) + i fi”(E) vm(E) = E * fi”(E) e v. Kramers -Kronig : : fi’(E) <-> fi”(E)

Resonance Scattering vs Xanes

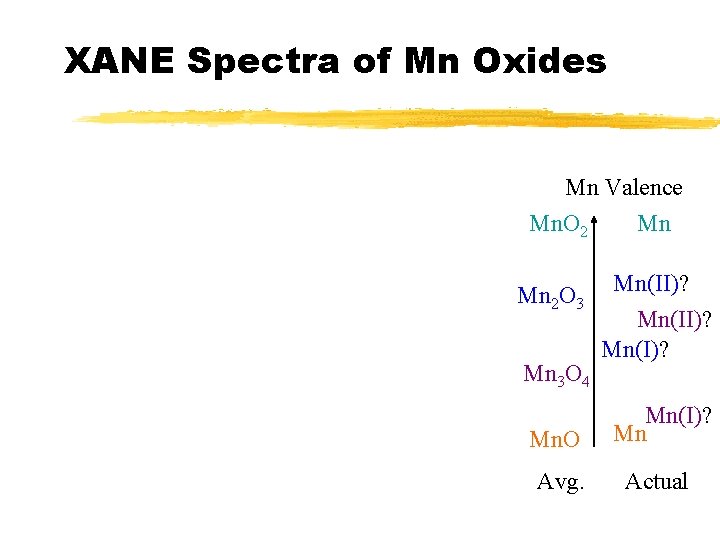
XANE Spectra of Mn Oxides Mn Valence Mn. O 2 Mn Mn 2 O 3 Mn 3 O 4 Mn(II)? Mn(I)? Mn. O Mn(I)? Mn Avg. Actual

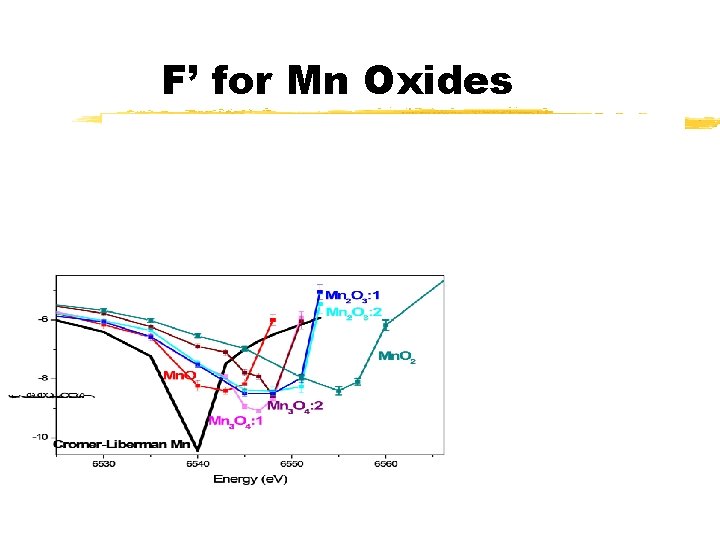
F’ for Mn Oxides

Why Resonance Scattering? v. Sensitive to a specific crystallographic phase. (e. g. , can investigate Fe. O layer growing on metallic Fe. ) v. Sensitive to a specific crystallographic site in a phase. (e. g. , can investigate the tetrahedral and the octahedral site of Mn 3 O 4)

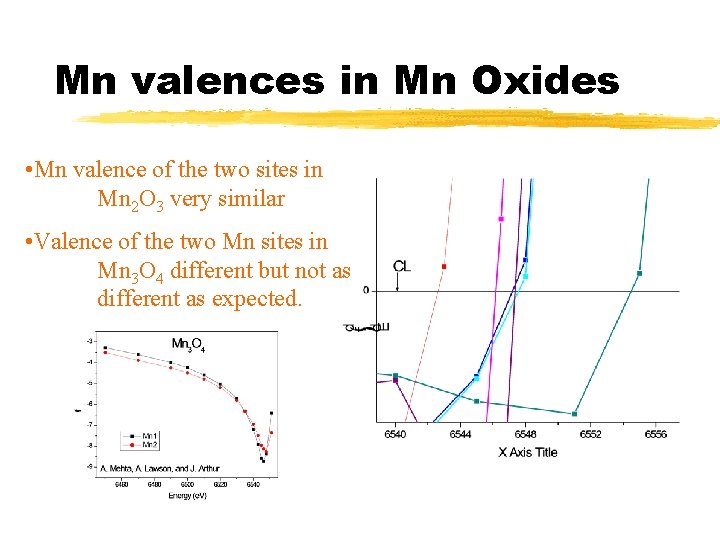
Mn valences in Mn Oxides • Mn valence of the two sites in Mn 2 O 3 very similar • Valence of the two Mn sites in Mn 3 O 4 different but not as different as expected.