Diffraction Xray Neutron Electron Diffraction An atom in


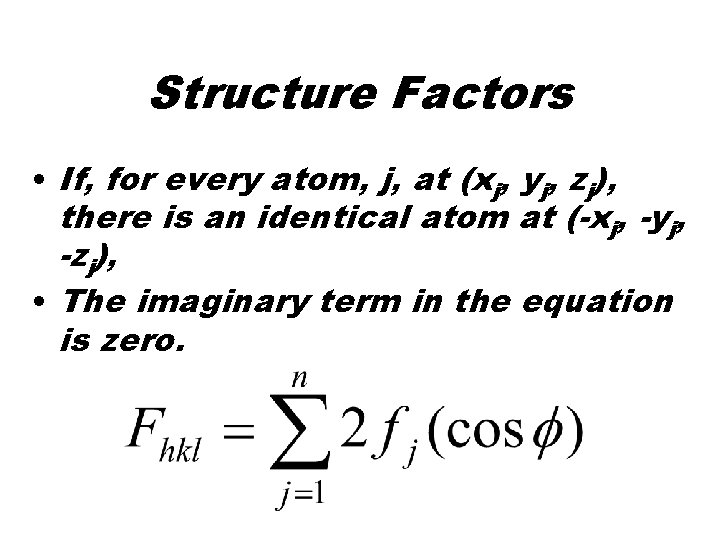








- Slides: 18

Diffraction X-ray Neutron Electron

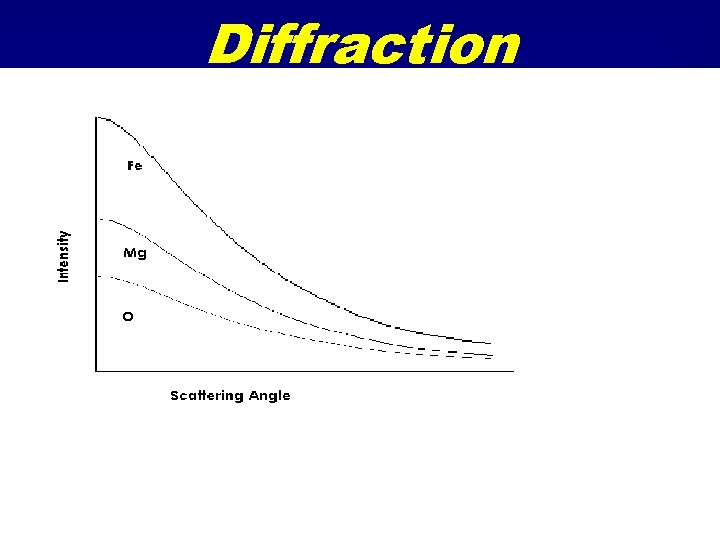
Diffraction • An atom in space will elastically scatter X-rays as a function of angle. • It will behave as a point source, but the intensity will decrease with angle. • The intensity is calculated from theoretical wave functions for the electron distribution and given as a polynomial expression called the scattering factor (f ).

Diffraction

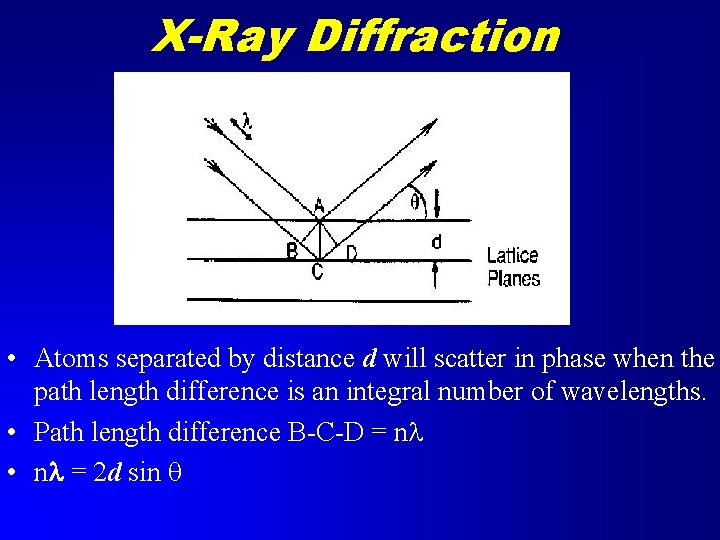
X-Ray Diffraction • Atoms separated by distance d will scatter in phase when the path length difference is an integral number of wavelengths. • Path length difference B-C-D = nl • nl = 2 d sin q

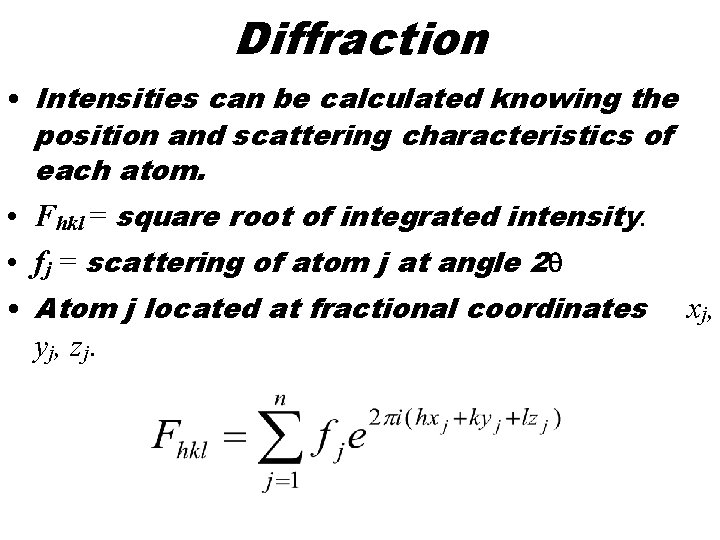
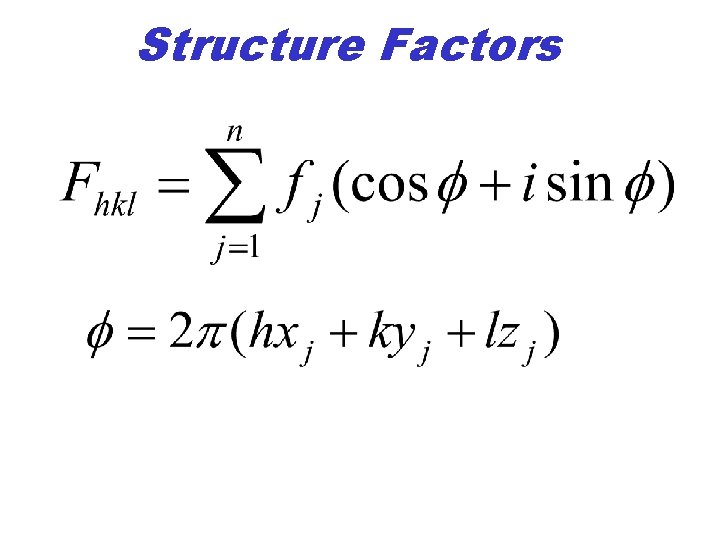
Diffraction • Intensities can be calculated knowing the position and scattering characteristics of each atom. • Fhkl = square root of integrated intensity. • fj = scattering of atom j at angle 2 q • Atom j located at fractional coordinates y j , zj. x j,

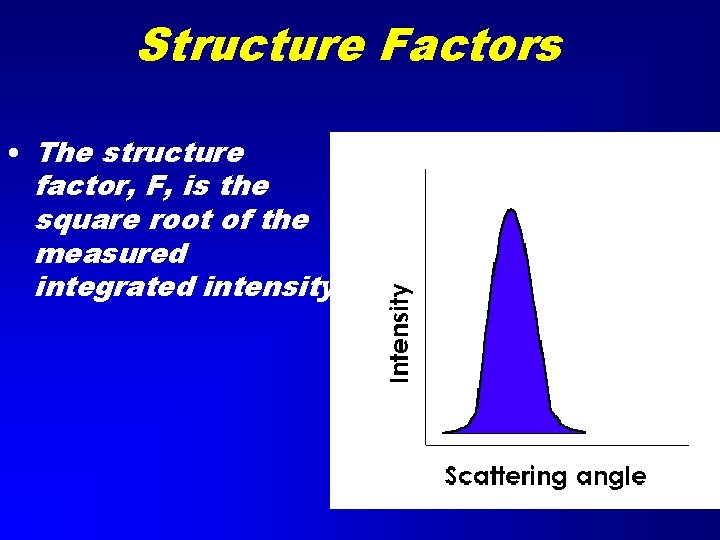
Structure Factors • The structure factor, F, is the square root of the measured integrated intensity.

Structure Factors
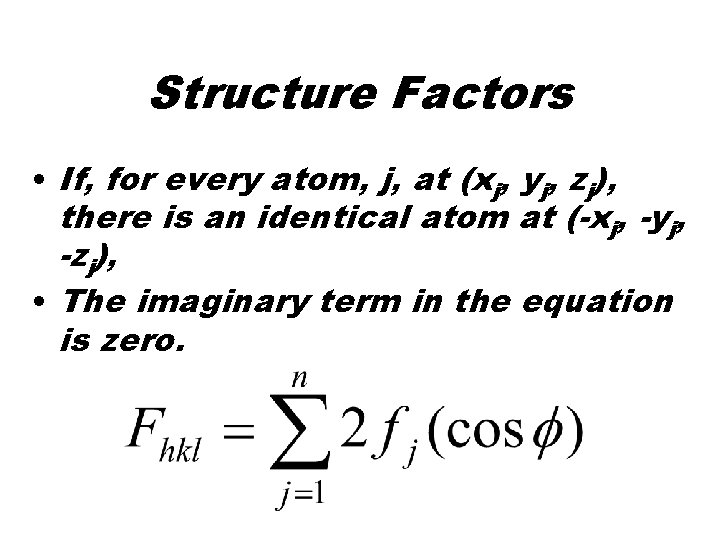
Structure Factors • If, for every atom, j, at (xj, yj, zj), there is an identical atom at (-xj, -yj, -zj), • The imaginary term in the equation is zero.

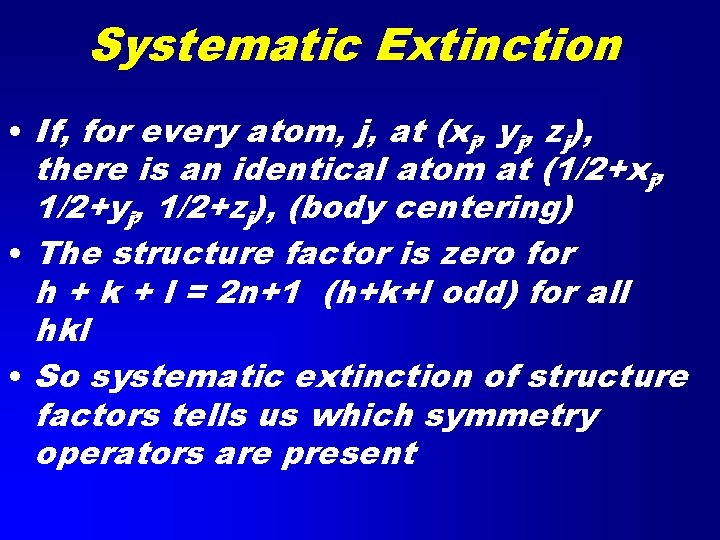
Systematic Extinction • If, for every atom, j, at (xj, yj, zj), there is an identical atom at (1/2+xj, 1/2+yj, 1/2+zj), (body centering) • The structure factor is zero for h + k + l = 2 n+1 (h+k+l odd) for all hkl • So systematic extinction of structure factors tells us which symmetry operators are present

Systematic Extinction • Lattice centering Operations – For all hkl – F: hkl all even or all odd – I: h+k+l = 2 n – A: k+l = 2 n – B: h+l = 2 n – C: h+k = 2 n

Systematic Extinction • Glide planes – a glide – b-glide – n-glide – a-glide – c-glide – n-glide – b-glide – c-glide – n-glide normal normal normal to to to c: c: c: b: b: b: a: a: a: for for for hk 0, h = 2 n hk 0, k = 2 n hk 0, h+k = 2 n h 0 l, h = 2 n h 0 l, l = 2 n h 0 l, h+l = 2 n 0 kl, k = 2 n 0 kl, l = 2 n 0 kl, k+l = 2 n

Reciprocal Lattice • The reciprocal lattice is a mathematical construct of points each corresponding to a given Miller index, hkl. • It is a three dimensional lattice where the nodes are diffracted intensities and the spacing between points is inversely proportional to the unit cell parameters in real space.

Reciprocal Lattice • Reciprocal axes are denoted – a*, b*, c*, a*, b*, g* • For Orthorhombic – a* = 1/a ; b* = 1/b ; c* = 1/c • Scale is arbitrary

Wadsleyite Imma hk 0 a = 5. 7Å b = 11. 5 Å c = 8. 3 Å

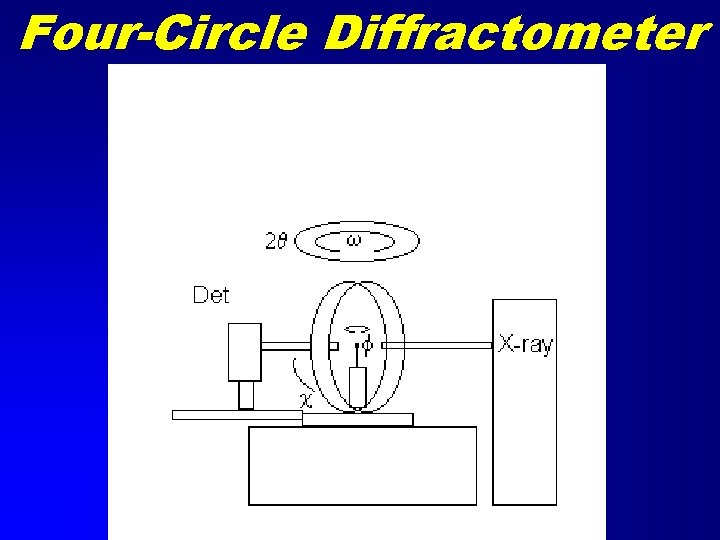
Four-Circle Diffractometer

Four-Circle Diffractometer

Four-Circle Diffractometer

Diffraction Experiment • • • Mount Crystal (~100 mm) Rotation photograph Index and obtain matrix Refine unit cell parameters (1/104) Measure Intensities (1000 - 10000) Determine or refine atom position and displacement parameters