MOLECULAR DIAGNOSIS OF NSCLCWHATS NEW Dr Anurag Mehta





























- Slides: 46

MOLECULAR DIAGNOSIS OF NSCLCWHAT’S NEW Dr. Anurag Mehta Director Lab & Molecular Services RGCI & RC

Established 1. Genome directed therapy is the standard treatment for “Metastatic Lung Adenocarcinoma”. 2. Several driver alterations are druggable. 3. Three targets are actionable by FDA approved drugs. ROS 1 is a new addition 4. Currently, no targeted therapy is available for SCC & SCLC

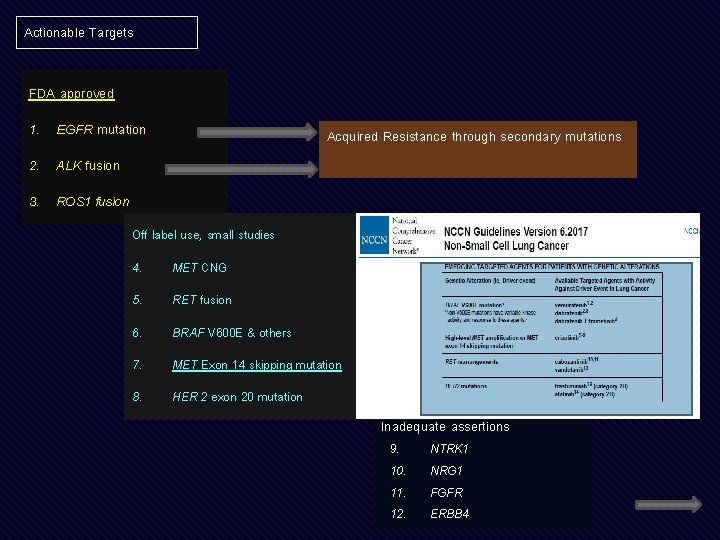
Actionable Targets FDA approved 1. EGFR mutation Acquired Resistance through secondary mutations 2. ALK fusion 3. ROS 1 fusion Off label use, small studies 4. MET CNG 5. RET fusion 6. BRAF V 600 E & others 7. MET Exon 14 skipping mutation 8. HER 2 exon 20 mutation Inadequate assertions 9. 10. 11. 12. NTRK 1 NRG 1 FGFR ERBB 4

What’s new? 1. ROS 1 fusion is a new addition as a target by a FDA approved drug. (Crizotinib. Mar 2016) 2. No designated companion diagnostic. 3. FISH is the established method. Unlike in ALK; FISH is easier to interpret. 4. RNA methods ( RT - PCR, anchored multiplexed PCR / RACE PCR) 5. Next generation DNA sequencing 6. IHC has good sensitivity but lower specificity. PPV is not satisfactory. Can be used as a screening tool but confirmation with FISH is necessary. IHC is not binary. Grading is necessary but subjective. Process is not harmonized.

What’s new? 1. New draft guidelines from CAP, IASLC & AMP • Optimize all phases of laboratory testing • Testing for secondary mutations leading to acquired resistance • Recommendations for selection of appropriate testing methodology/ platform(s) & in special context. • Also have recommendations for expanded testing- other than main 3. • Tissue proficient testing – multiplexed sequence based testing.


What’s new? 2016 statement Recommendation: Physician’s should use molecular testing for the appropriate genetic targets on either primary or metastatic lung lesions to guide initial therapy selection. Explanation To determine EGFR and ALK status for initial treatment selection, primary tumors or metastatic lesions are equally suitable for testing.

What’s new? 2016 statement Explanation Recommendation: In some clinical settings in which tissue is limited and/or insufficient for molecular testing, physicians may utilize a mutation - specific IHC assay for EGFR testing New Recommendation statement Recommendation: When performing ALK testing, physicians can utilize IHC as an equivalent alternative to FISH. No scientific need to perform both methods New Recommendation Statement

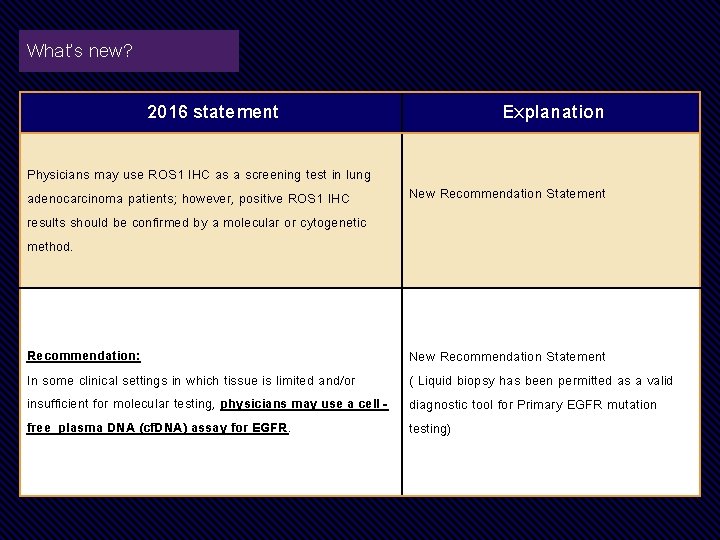
What’s new? 2016 statement Physicians may use ROS 1 IHC as a screening test in lung adenocarcinoma patients; however, positive ROS 1 IHC results should be confirmed by a molecular or cytogenetic method. Recommendation: In some clinical settings in which tissue is limited and/or insufficient for molecular testing, physicians may use a cell free plasma DNA (cf. DNA) assay for EGFR. Explanation New Recommendation Statement ( Liquid biopsy has been permitted as a valid diagnostic tool for Primary EGFR mutation testing)

What’s new? 2016 statement Explanation Strong Recommendation: In lung adenocarcinoma patients who harbor sensitizing EGFR mutations and have progressed after treatment with an EGFR –targeted TKI, physicians must use EGFR T 790 M mutational testing when selecting patients for third generation EGFR – targeted therapy New Recommendations Recommendation: Laboratories testing for EGFR T 790 M mutation in patients with acquired resistance to EGFR –targeted kinase inhibitors should deploy assays capable of detecting EGFR T 790 M mutations in as little as 4% of viable cells (2% of EGFR alleles).

What’s new? 2016 statement Expert Consensus Opinion: Physicians may use cell free plasma DNA (cf. DNA) methods to identify EGFR T 790 M mutations in lung adenocarcinoma patients with progression or acquired resistance to EGFR -targeted tyrosine kinase inhibitors; testing of the tumor sample is recommended if the plasma result is negative. Expert Consensus Opinion: Multiplexed genetic sequencing panels are preferred over multiple single gene tests to identify other treatment options beyond EGFR, ALK and ROS 1. Expansion New statements

What’s new? 2016 statement No Recommendation: There is currently insufficient evidence to support a recommendation for or against routine testing for ALK mutational status for lung adenocarcinoma patients with sensitizing ALK mutations who have progressed after treatment with an ALK targeted tyrosine kinase inhibitor. Expert Consensus Opinion: BRAF, RET, MET, HER 2 & KRAS molecular testing is currently not indicated as a routine stand-alone assay outside the context of a clinical trial. As part of larger testing panels performed either initially or when routine EGFR, ALK, and ROS 1 testing are negative, it is appropriate to include BRAF, RET, MET, HER 2 & KRAS in the panel done as an initial test or to identify other treatment options. Expansion ALK acquired resistance testing is not mature for clinical usage New statement

Advances in molecular diagnostics 1. Mutation specific IHC for EGFR has been accepted in special setting. 2. Liquid biopsy as a primary assay for EGFR mutation is acceptable 3. IHC for ALK testing is adequate 4. Molecular testing beyond three!! 5. Multiple marker testing in a single experiment 6. Testing for resistance mutation in EGFR (T 790 M)- role of liquid biopsy

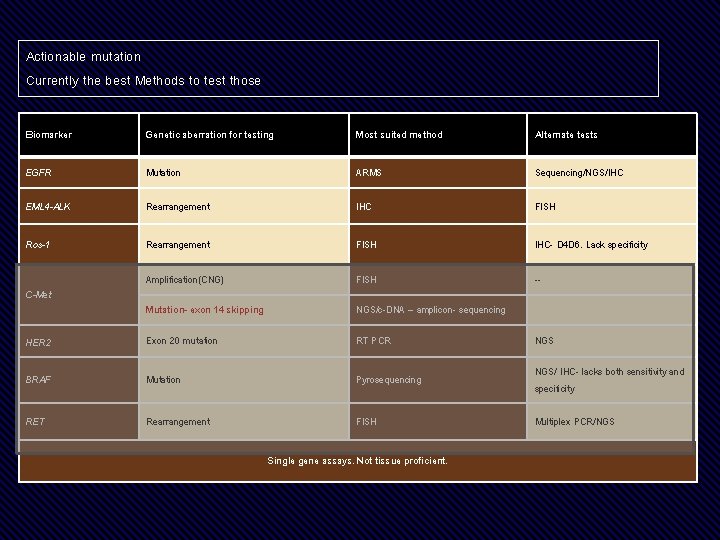
Actionable mutation Currently the best Methods to test those Biomarker Genetic aberration for testing Most suited method Alternate tests EGFR Mutation ARMS Sequencing/NGS/IHC EML 4 -ALK Rearrangement IHC FISH Ros-1 Rearrangement FISH IHC- D 4 D 6. Lack specificity Amplification(CNG) FISH -- Mutation- exon 14 skipping NGS/c-DNA – amplicon- sequencing HER 2 Exon 20 mutation RT PCR NGS BRAF Mutation Pyrosequencing NGS/ IHC- lacks both sensitivity and specificity RET Rearrangement FISH Multiplex PCR/NGS C-Met Single gene assays. Not tissue proficient.

NGS panels preferred over single gene tests 1. Results are quicker 2. Overcomes issue of sample availability 3. Enables expanded testing beyond EGFR, ALK, ROS 1 4. Can help patients find appropriate treatment/ clinical trials.


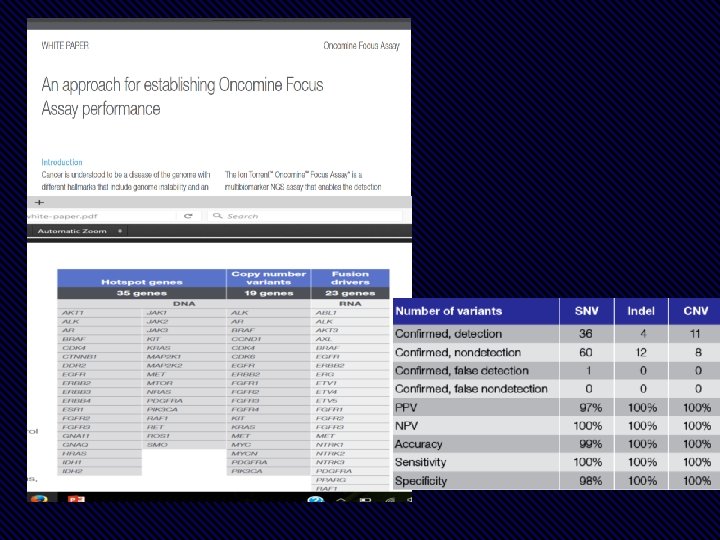
Targeted NGS “Oncomine Dx Target Test Panel” All driver mutations are analyzed in 1 experiment.





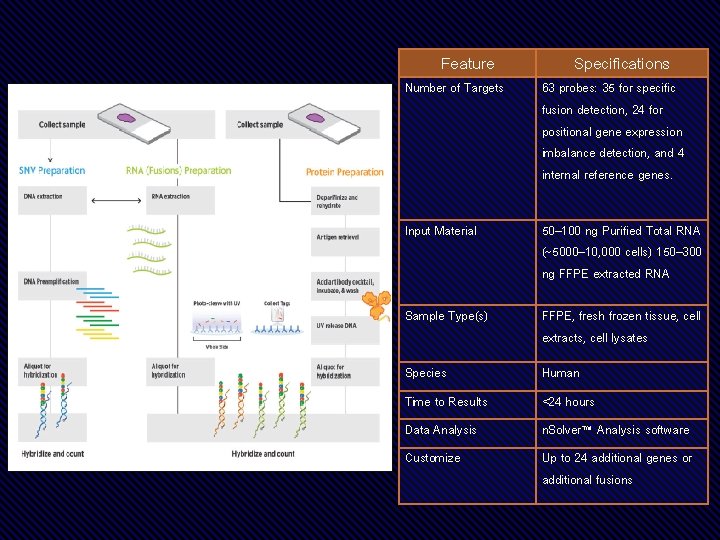
• Detect 104 actionable SNV and INDELS, 63 lung gene fusions, and up to 28 total and phospho-proteins • Low sample inputs; as little as 5 ng DNA, 50 ng RNA, and 250 ng protein or just 2 FFPE slides • Integrated SNV, Fusion, and protein measurements allow for simplified data analysis • Compatible with many sample types, including cell or tissue lysate and FFPE

Feature Specifications Number of Targets 63 probes: 35 for specific fusion detection, 24 for positional gene expression imbalance detection, and 4 internal reference genes. Input Material 50– 100 ng Purified Total RNA (~5000– 10, 000 cells) 150– 300 ng FFPE extracted RNA Sample Type(s) FFPE, fresh frozen tissue, cell extracts, cell lysates Species Human Time to Results <24 hours Data Analysis n. Solver™ Analysis software Customize Up to 24 additional genes or additional fusions

What else is new? 1. Testing on progression- acquired resistance 2. Biomarker testing for immunotherapy

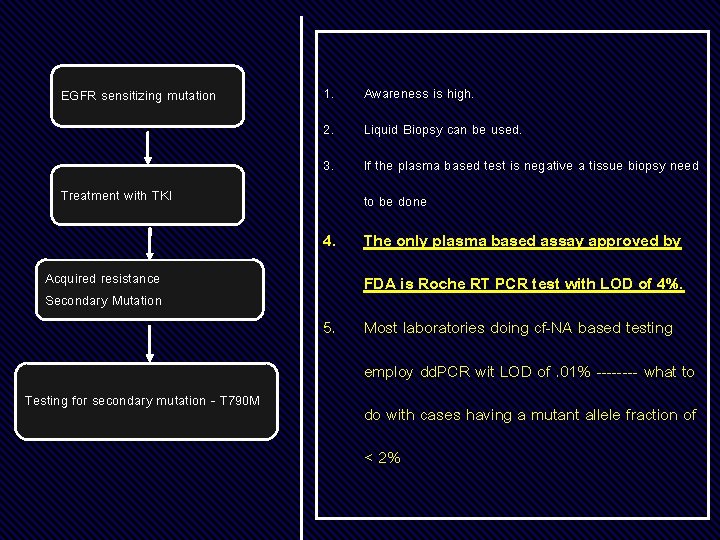
Testing on progression

EGFR sensitizing mutation 1. Awareness is high. 2. Liquid Biopsy can be used. 3. If the plasma based test is negative a tissue biopsy need Treatment with TKI to be done 4. Acquired resistance Secondary Mutation The only plasma based assay approved by FDA is Roche RT PCR test with LOD of 4%. 5. Most laboratories doing cf-NA based testing employ dd. PCR wit LOD of. 01% ---- what to Testing for secondary mutation - T 790 M do with cases having a mutant allele fraction of < 2%

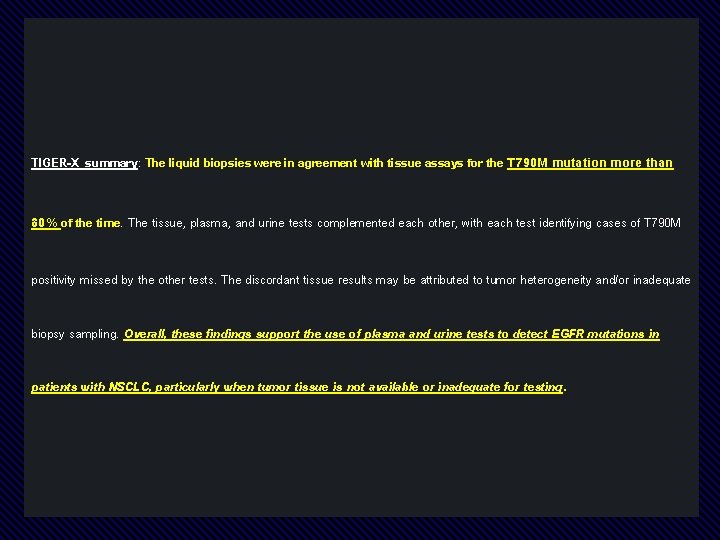
TIGER-X summary: The liquid biopsies were in agreement with tissue assays for the T 790 M mutation more than 80% of the time. The tissue, plasma, and urine tests complemented each other, with each test identifying cases of T 790 M positivity missed by the other tests. The discordant tissue results may be attributed to tumor heterogeneity and/or inadequate biopsy sampling. Overall, these findings support the use of plasma and urine tests to detect EGFR mutations in patients with NSCLC, particularly when tumor tissue is not available or inadequate for testing.

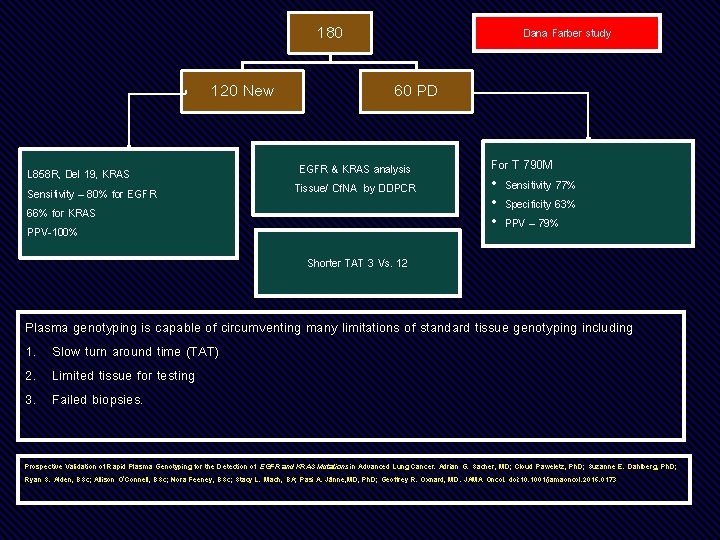
180 120 New L 858 R, Del 19, KRAS Sensitivity – 80% for EGFR 66% for KRAS PPV-100% Dana Farber study 60 PD EGFR & KRAS analysis Tissue/ Cf. NA by DDPCR For T 790 M • Sensitivity 77% • Specificity 63% • PPV – 79% Shorter TAT 3 Vs. 12 Plasma genotyping is capable of circumventing many limitations of standard tissue genotyping including 1. Slow turn around time (TAT) 2. Limited tissue for testing 3. Failed biopsies. Prospective Validation of Rapid Plasma Genotyping for the Detection of EGFR and KRAS Mutations in Advanced Lung Cancer. Adrian G. Sacher, MD; Cloud Paweletz, Ph. D; Suzanne E. Dahlberg, Ph. D; Ryan S. Alden, BSc; Allison O’Connell, BSc; Nora Feeney, BSc; Stacy L. Mach, BA; Pasi A. Jänne, MD, Ph. D; Geoffrey R. Oxnard, MD. JAMA Oncol. doi: 10. 1001/jamaoncol. 2016. 0173

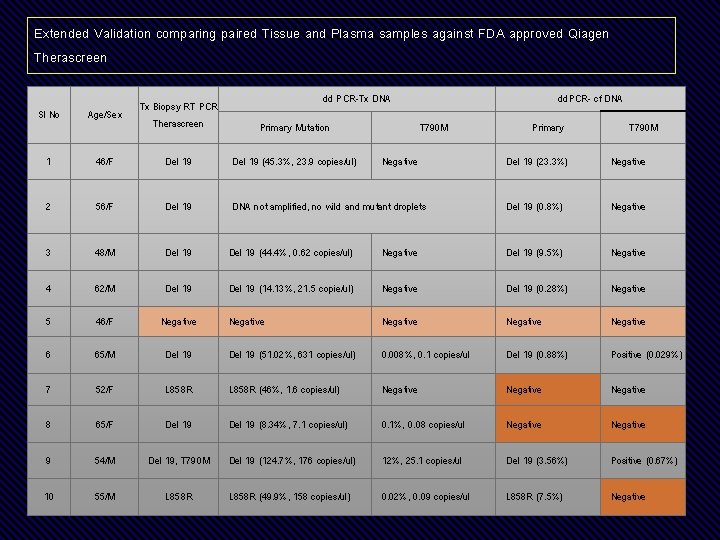
Extended Validation comparing paired Tissue and Plasma samples against FDA approved Qiagen Therascreen dd PCR-Tx DNA Sl No Age/Sex Tx Biopsy RT PCR Therascreen 1 46/F Del 19 (45. 3%, 23. 9 copies/ul) 2 56/F Del 19 3 48/M 4 Primary Mutation dd. PCR- cf DNA T 790 M Negative Primary T 790 M Del 19 (23. 3%) Negative DNA not amplified, no wild and mutant droplets Del 19 (0. 8%) Negative Del 19 (44. 4%, 0. 62 copies/ul) Negative Del 19 (9. 5%) Negative 62/M Del 19 (14. 13%, 21. 5 copie/ul) Negative Del 19 (0. 28%) Negative 5 46/F Negative Negative 6 65/M Del 19 (51. 02%, 631 copies/ul) 0. 008%, 0. 1 copies/ul Del 19 (0. 88%) Positive (0. 029%) 7 52/F L 858 R (46%, 1. 6 copies/ul) Negative 8 65/F Del 19 (8. 34%, 7. 1 copies/ul) 0. 1%, 0. 08 copies/ul Negative 9 54/M Del 19, T 790 M Del 19 (124. 7%, 176 copies/ul) 12%, 25. 1 copies/ul Del 19 (3. 56%) Positive (0. 67%) 10 55/M L 858 R (49. 9%, 158 copies/ul) 0. 02%, 0. 09 copies/ul L 858 R (7. 5%) Negative

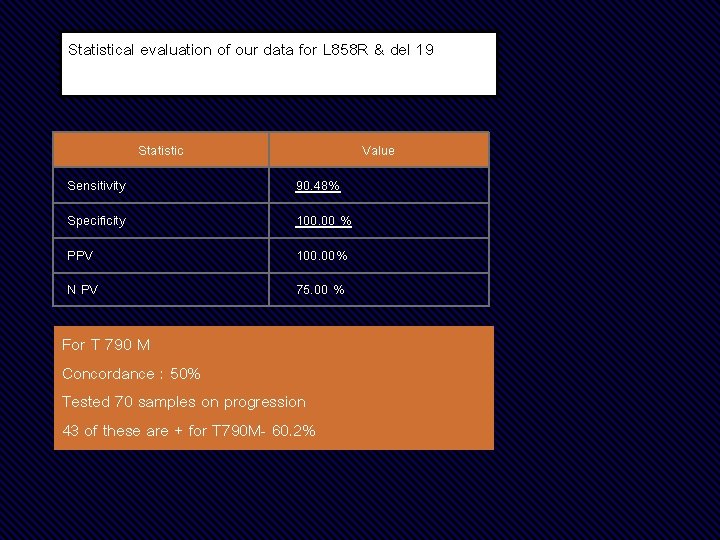
Statistical evaluation of our data for L 858 R & del 19 Statistic Value Sensitivity 90. 48% Specificity 100. 00 % PPV 100. 00% N PV 75. 00 % For T 790 M Concordance : 50% Tested 70 samples on progression 43 of these are + for T 790 M- 60. 2%

2. To detect secondary mutation as cause of acquired resistance

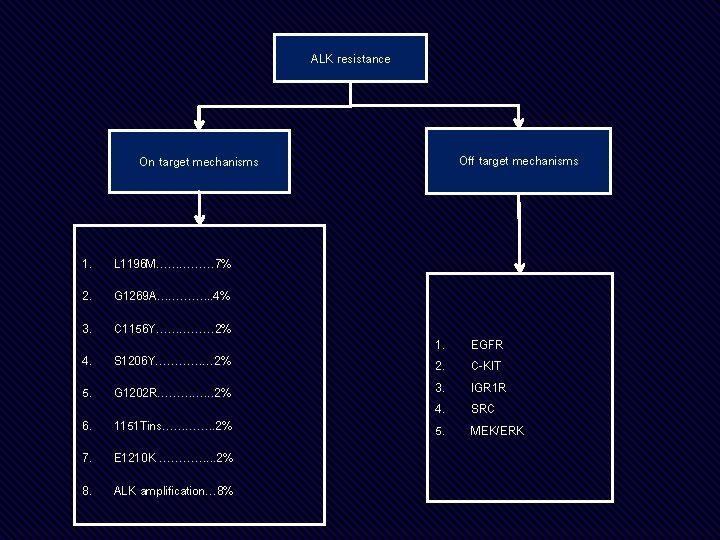
ALK rearrangement Treat with a cognate TKI Acquired Resistance The response to next generation of TKI is variable dependent on nature of genetic alteration Is there a role for secondary mutation testing

ALK resistance Off target mechanisms On target mechanisms 1. L 1196 M…………… 7% 2. G 1269 A…………. . . 4% 3. C 1156 Y…………… 2% 4. S 1206 Y…………… 2% 5. G 1202 R…………. . . 2% 6. 1151 Tins…………. . 2% 7. E 1210 K …………. . . 2% 8. ALK amplification… 8% 1. 2. 3. 4. 5. EGFR C-KIT IGR 1 R SRC MEK/ERK


Experimental. Evidence for Mutation testing not considered enough

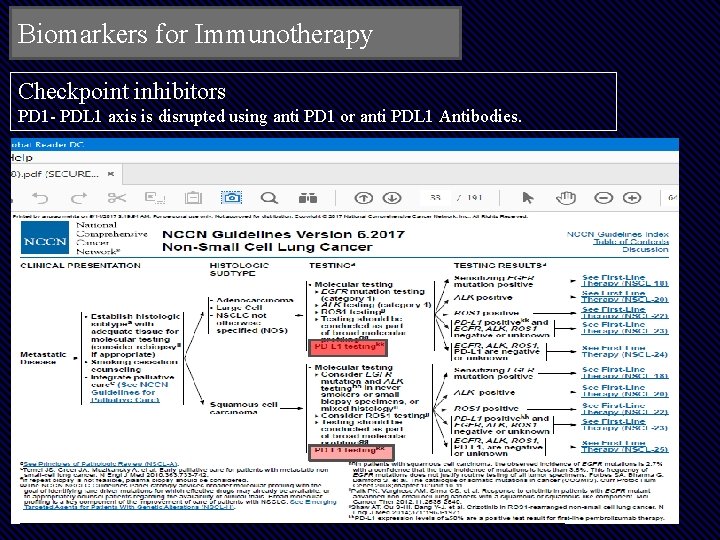
Biomarkers for Immunotherapy Checkpoint inhibitors PD 1 - PDL 1 axis is disrupted using anti PD 1 or anti PDL 1 Antibodies.

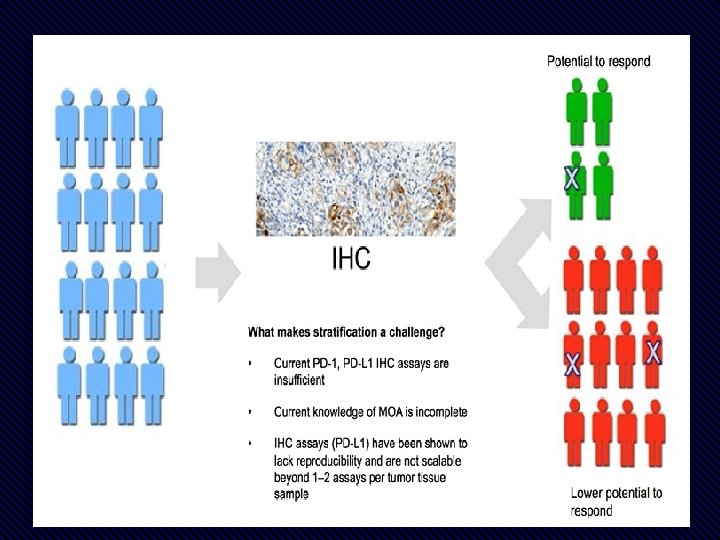
1. The existing biomarker test used is identifying expression of PDL 1 on tumor cells & also in microenvironment. 2. Problems • Several assays on several platforms for different drugs • Clinical validity: PPV & NPV is low

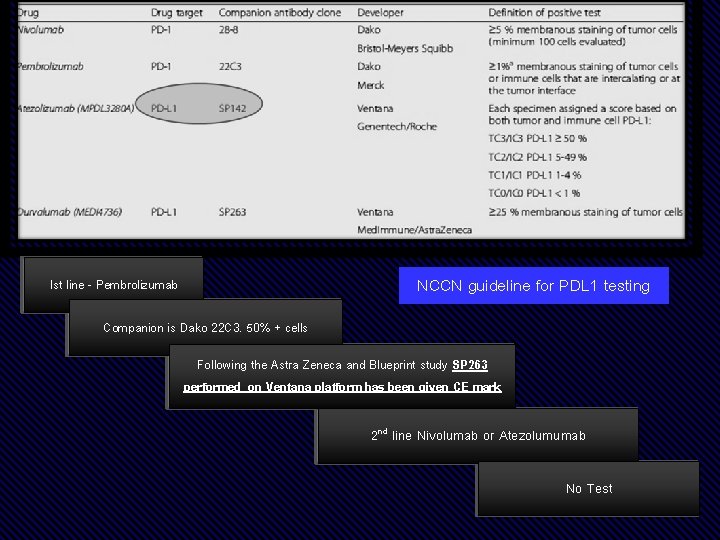
NCCN guideline for PDL 1 testing Ist line -- Pembrolizumab Companion isis Dako 22 C 3. 50% ++ cells Following the Astra Zeneca and Blueprint study SP 263 performed on on Ventana platform has been given CE CE mark 22 ndnd line Nivolumab oror Atezolumumab No No Test

Media Release Roche’s VENTANA PD-L 1 (SP 263) Assay gains CE label expansion to inform treatment decisions in lung cancer patients being considered for KEYTRUDA (pembrolizumab) immunotherapy Lung cancer remains the leading cause of cancer deaths, with nearly 1. 6 million deaths worldwide. PD-L 1 is a protein involved in the suppression of the immune system, which can impact the body’s ability to fight cancer. The VENTANA PD-L 1 (SP 263) Assay 1 is now available for countries accepting the CE mark to identify untreated and previously treated metastatic non-small cell lung cancer (m. NSCLC) patients eligible for KEYTRUDA immunotherapy.

An effort is on to improve biomarker testing for immunotherapy- Planned through • Total Mutation Burden • Immune gene profiling from tumor tissue • Looking for BRCA 1 - 2, POLE, POLD mutations and MSI in a tumor. • Multiplexing of IHC

Conclusions 1. Major advances 2. Rationalization of molecular testing 3. Testing beyond 3 approved target is encouraged and many physicians practice it. 4. Multiplexed, multi gene sequencing is being advocated. 5. Liquid biopsy is a new tool • For secondary mutation detection in patients progressing on 1 st and 2 nd line EGFR TKIs • For primary predictive biomarker testing. . 6. Immunotherapy biomarker testing is being reorganized.

Thank you for your patience

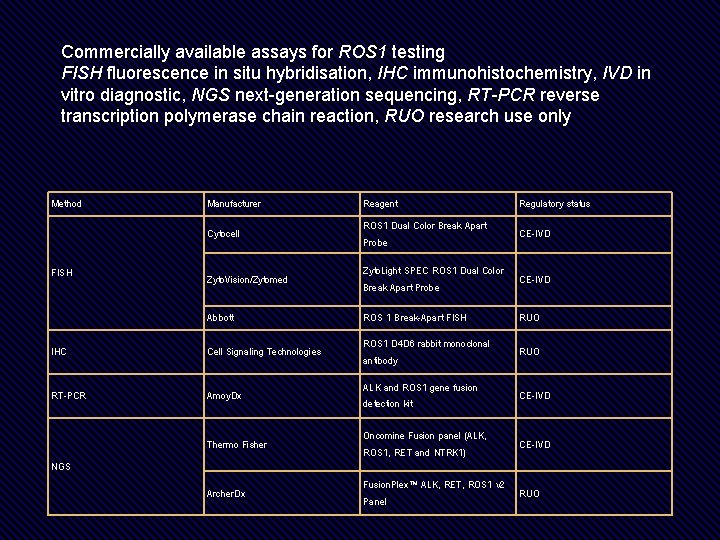
Commercially available assays for ROS 1 testing FISH fluorescence in situ hybridisation, IHC immunohistochemistry, IVD in vitro diagnostic, NGS next-generation sequencing, RT-PCR reverse transcription polymerase chain reaction, RUO research use only Method Manufacturer Reagent Regulatory status Cytocell ROS 1 Dual Color Break Apart Probe CE-IVD Zyto. Vision/Zytomed Zyto. Light SPEC ROS 1 Dual Color Break Apart Probe CE-IVD Abbott ROS 1 Break-Apart FISH RUO IHC Cell Signaling Technologies ROS 1 D 4 D 6 rabbit monoclonal antibody RUO RT-PCR Amoy. Dx ALK and ROS 1 gene fusion detection kit CE-IVD Thermo Fisher Oncomine Fusion panel (ALK, ROS 1, RET and NTRK 1) CE-IVD Archer. Dx Fusion. Plex™ ALK, RET, ROS 1 v 2 Panel RUO FISH NGS



 Snv
Snv How to write a diary entry for english
How to write a diary entry for english Anurag acharya google
Anurag acharya google Refeeding syndrome
Refeeding syndrome Anurag lehtonen
Anurag lehtonen Quantanization
Quantanization Collaborative nursing interventions
Collaborative nursing interventions Medical diagnosis and nursing diagnosis difference
Medical diagnosis and nursing diagnosis difference Types of nursing diagnosis
Types of nursing diagnosis Types of nursing diagnoses
Types of nursing diagnoses Perbedaan diagnosis gizi dan diagnosis medis
Perbedaan diagnosis gizi dan diagnosis medis Covalent bond
Covalent bond Ionic covalent metallic
Ionic covalent metallic Zinc oxide + nitric acid → zinc nitrate + water
Zinc oxide + nitric acid → zinc nitrate + water Mehta cast
Mehta cast G.v.k. rao committee in tamil
G.v.k. rao committee in tamil Nigel price md
Nigel price md Manoj mehta ficci
Manoj mehta ficci Principles of electrical machines vk mehta
Principles of electrical machines vk mehta Minesh mehta md
Minesh mehta md Tanya singh husband
Tanya singh husband Mehta hydraulics
Mehta hydraulics Amari mehta
Amari mehta Amita mehta nasa
Amita mehta nasa Suri auto pvt ltd
Suri auto pvt ltd Rakesh mehta nsdl
Rakesh mehta nsdl Chet mehta
Chet mehta Mehta
Mehta Balwant rai mehta committee
Balwant rai mehta committee Nimai mehta
Nimai mehta Sohin mehta
Sohin mehta Alexandra mehta
Alexandra mehta Febrile convulsions
Febrile convulsions Brijesh mehta md
Brijesh mehta md Nachiket mehta
Nachiket mehta Split speech punctuation
Split speech punctuation New york, new jersey, pennsylvania, and delaware
New york, new jersey, pennsylvania, and delaware Fresh oil, new wine scripture
Fresh oil, new wine scripture Movie theaters new hartford ny
Movie theaters new hartford ny Characteristics of the articles of confederation
Characteristics of the articles of confederation New-old approach to creating new ventures
New-old approach to creating new ventures New marketing realities
New marketing realities Njbta
Njbta New classical macroeconomics
New classical macroeconomics Chapter 16 toward a new heaven and a new earth
Chapter 16 toward a new heaven and a new earth Neil thisse is a loyalist who fled the colonies
Neil thisse is a loyalist who fled the colonies New classical and new keynesian macroeconomics
New classical and new keynesian macroeconomics