STATO DI SVILUPPO DELLACCUMULO ENERGETICO PER VIA ELETTROCHIMICA














- Slides: 27

STATO DI SVILUPPO DELL’ACCUMULO ENERGETICO PER VIA ELETTROCHIMICA LE BATTERIE AL LITIO Bruno Scrosati Laboratory for Advanced Batteries and Fuel Cell Technology Dipartimento di Chimica Centro Hydro-ECO SAPIENZA Università di Roma

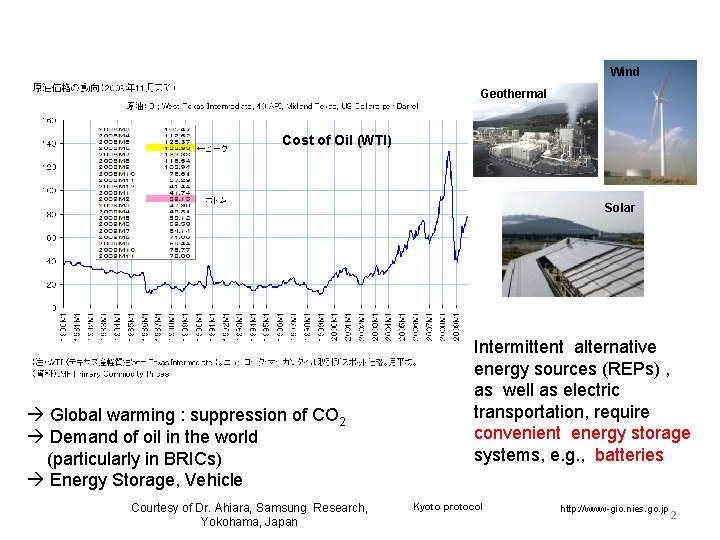
Research background Wind Geothermal Cost of Oil (WTI) Solar Global warming : suppression of CO 2 Demand of oil in the world (particularly in BRICs) Energy Storage, Vehicle Courtesy of Dr. Ahiara, Samsung Research, Yokohama, Japan Intermittent alternative energy sources (REPs) , as well as electric transportation, require convenient energy storage systems, e. g. , batteries Kyoto protocol http: //www-gio. nies. go. jp 2

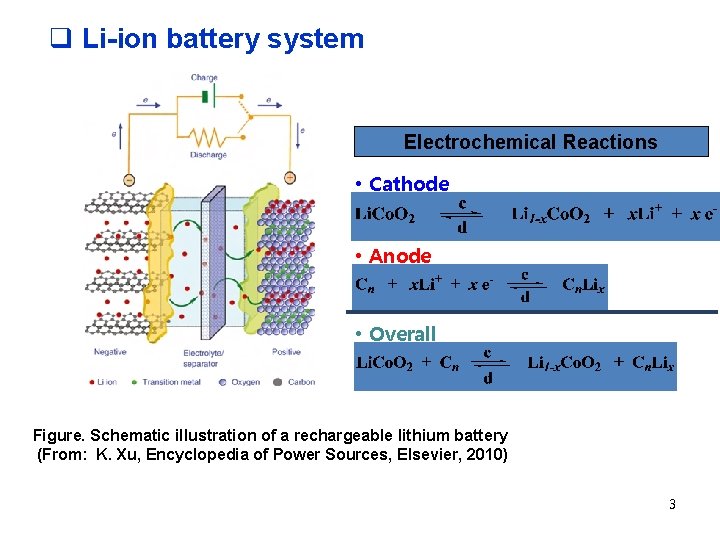
q Li-ion battery system Electrochemical Reactions • Cathode • Anode • Overall Figure. Schematic illustration of a rechargeable lithium battery (From: K. Xu, Encyclopedia of Power Sources, Elsevier, 2010) 3

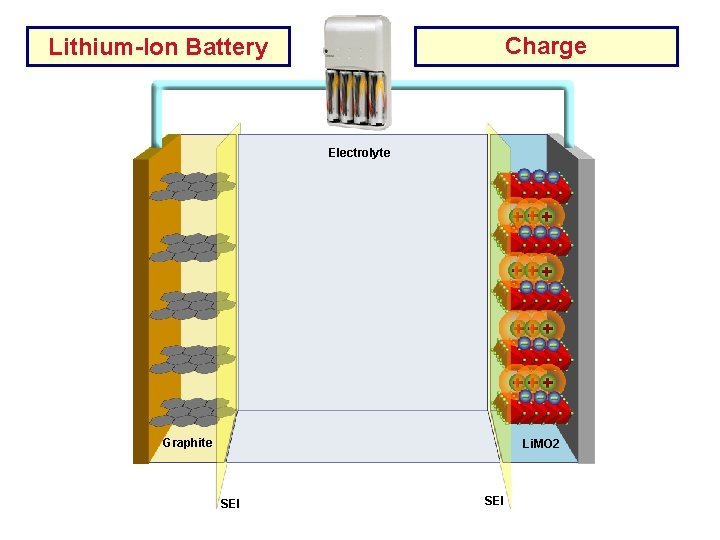
Charge Lithium-Ion Battery Electrolyte Cu Current AL Current Collector Graphite Li. MO 2 SEI

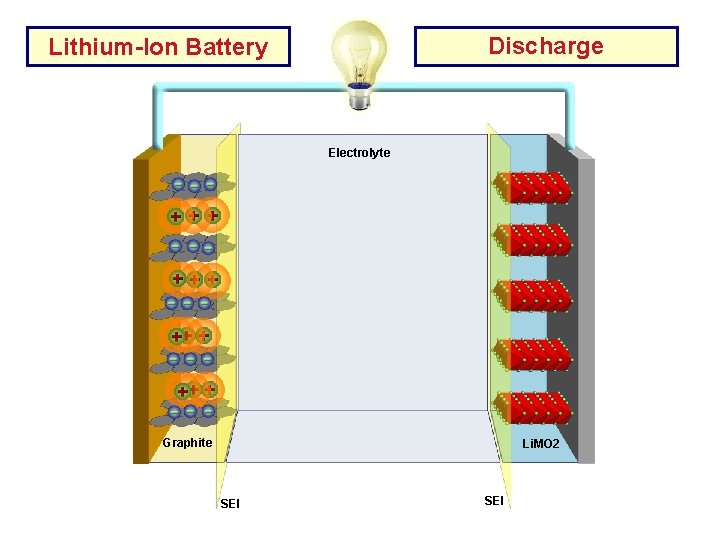
Discharge Lithium-Ion Battery Electrolyte Cu Current AL Current Collector Graphite Li. MO 2 SEI

Lithium Batteries Lithium batteries: high energy density (3 times leadacid). Power sources of choice for the consumer electronics market The application of lithium batteries spans beyond the electronics market

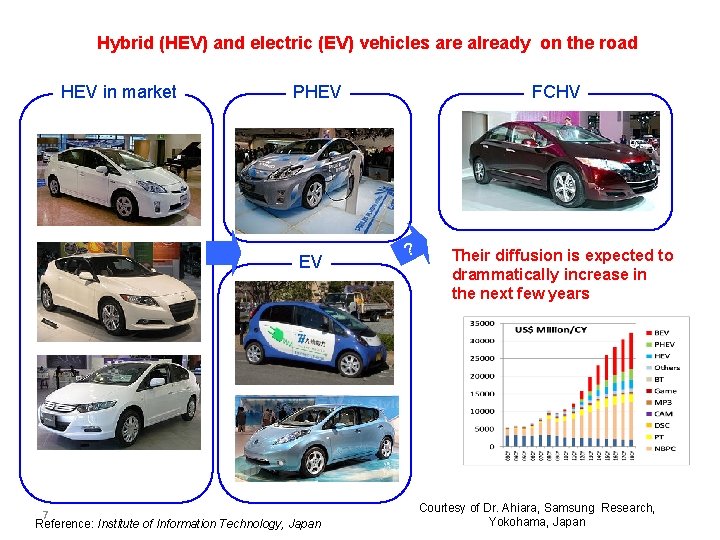
HEV, EV and FCV in Japan Hybrid (HEV) and electric (EV) vehicles are already on the road HEV in market PHEV EV 7 Reference: Institute of Information Technology, Japan FCHV ? Their diffusion is expected to drammatically increase in the next few years Courtesy of Dr. Ahiara, Samsung Research, Yokohama, Japan

Lithium Batteries Although lithium batteries are established commercial products further R&D is still required to improve their performance to meet the REP and. HEV-EV requirement Enhancement in safety, energy density and cost are needed!


THE SAFETY ISSUE

SAFETY Actions: Replacement of the oxygen releasing cathode material (Li. Co. O 2) with structurally stable alternative compounds, e. g. Li. Fe. PO 4 Replacement of the flammable liquid organic electrolyte with more stable materials, for example Polymer ionic conducting membranes

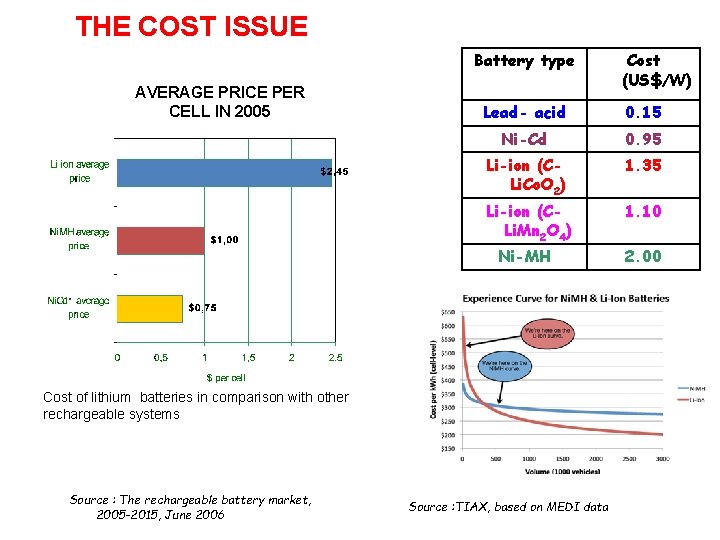
THE COST ISSUE Battery type AVERAGE PRICE PER CELL IN 2005 Lead- acid 0. 15 Ni-Cd 0. 95 Li-ion (CLi. Co. O 2) 1. 35 Li-ion (CLi. Mn 2 O 4) 1. 10 Ni-MH 2. 00 Cost of lithium batteries in comparison with other rechargeable systems Source : The rechargeable battery market, 2005 -2015, June 2006 Cost (US$/W) Source : TIAX, based on MEDI data

COST Actions: Replacement of the expensive cathode material (Li. Co. O 2) with low cost, abundant alternative compounds, ideally iron or sulfur – based cathodes

Cost Comparison of various raw materials for lithium secondary batteries. Cost US $/ton Cobalt Co Iron Fe (ore) Nickel Ni 41, 850 135 12, 350 Manga Copper Sulfur nese Cu (S) Mn (ore) 564 2, 770 28 Materials in use: Li. Co. O 2 (cathode) ; Cu (current collector) Alternative materials: Li. Fe. PO 4, Li. Mn 2 O 4, S (cathode) ; Stainless Steel (current collector)

THE ENERGY ISSUE Energy Density (Wh/kg) driving range (km) Middle size car (about 1, 100 kg) using presently available lithium batteries ( 150 Wh/kg) driving 250 km with a single charge 200 kg batteries Enhancement of about 2 -3 times in energy density is needed!

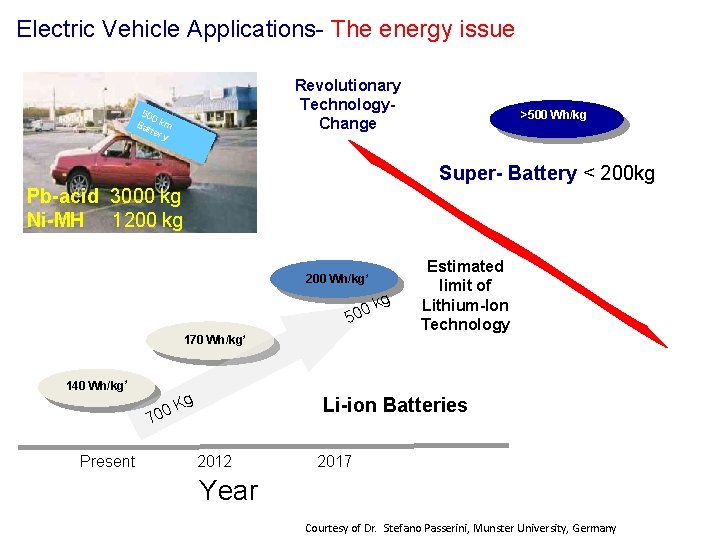
Electric Vehicle Applications- The energy issue Revolutionary Technology. Change 500 Ba km tter y >500 Wh/kg Super- Battery < 200 kg Pb-acid 3000 kg Ni-MH 1200 kg 200 Wh/kg* 0 50 kg Estimated limit of Lithium-Ion Technology 170 Wh/kg* 140 Wh/kg* Kg 0 0 Li-ion Batteries 7 Present 2012 2017 Year Courtesy of Dr. Stefano Passerini, Munster University, Germany

ENERGY DENSITY Actions: Replacement of the present electrode materials with alternative compounds having much higher values of specific capacity

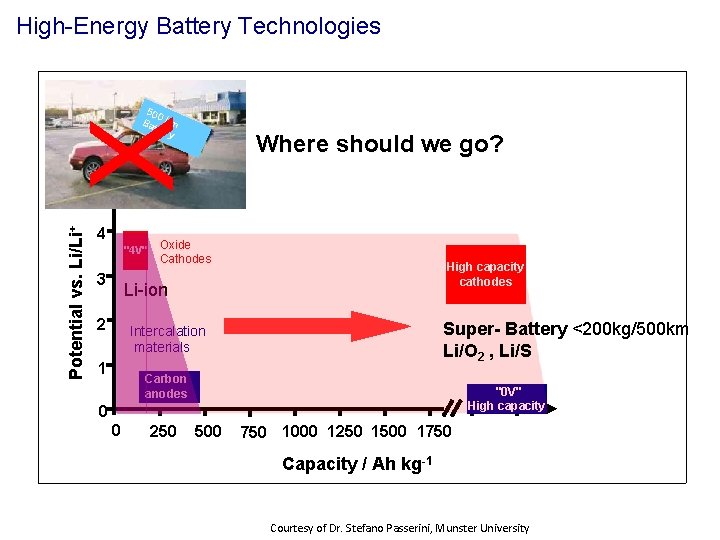
High-Energy Battery Technologies X 500 Ba km tter y 6 Where should we go? Potential vs. Li/Li+ 5 4 "4 V" 3 Oxide Cathodes High capacity cathodes Li-ion 2 Super- Battery <200 kg/500 km Li/O 2 , Li/S Intercalation materials 1 Carbon anodes "0 V" High capacity 0 0 250 500 750 1000 1250 1500 1750 Capacity / Ah kg-1 Courtesy of Dr. Stefano Passerini, Munster University

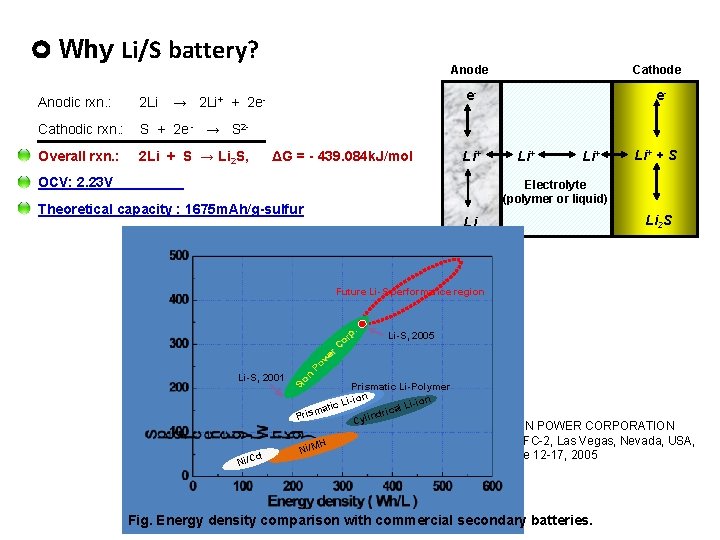
Why Li/S battery? Anodic rxn. : 2 Li Cathodic rxn. : S + 2 e - → S 2 - Overall rxn. : 2 Li + S → Li 2 S, Anode Cathode e- e- → 2 Li+ + 2 e- ΔG = - 439. 084 k. J/mol Li+ OCV: 2. 23 V Li+ Li+ + S Electrolyte (polymer or liquid) Theoretical capacity : 1675 m. Ah/g-sulfur Li 2 S Li Future Li-S performance region Li-S, 2001 d Ni/C on Si p. or C er w Po Li-S, 2005 Prismatic Li-Polymer i ion tic L l Lia a c m i r nd Pris Cyli Ni/M H SION POWER CORPORATION PBFC-2, Las Vegas, Nevada, USA, June 12 -17, 2005 Fig. Energy density comparison with commercial secondary batteries.

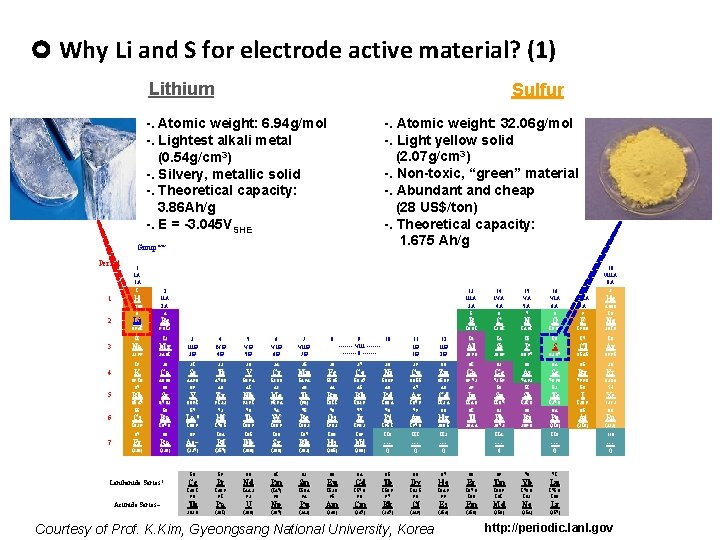
Why Li and S for electrode active material? (1) Lithium Sulfur -. Atomic weight: 32. 06 g/mol -. Light yellow solid (2. 07 g/cm 3) -. Non-toxic, “green” material -. Abundant and cheap (28 US$/ton) -. Theoretical capacity: 1. 675 Ah/g -. Atomic weight: 6. 94 g/mol -. Lightest alkali metal (0. 54 g/cm 3) -. Silvery, metallic solid -. Theoretical capacity: 3. 86 Ah/g -. E = -3. 045 VSHE Group** Period 1 IA 1 A 18 VIIIA 8 A 1 1 2 IIA 2 A H 1. 008 3 2 3 4 5 6 14 IVA 4 A 15 VA 5 A 16 VIA 6 A 17 VIIA 7 A 2 He 4. 003 10 4 5 6 7 8 9 Li Be B C N O F Ne 6. 941 9. 012 10. 81 12. 01 14. 01 16. 00 19. 00 20. 18 4 IVB 4 B 5 VB 5 B 6 VIB 6 B 7 VIIB 7 B 8 24. 31 3 IIIB 3 B 20 21 22 23 24 25 26 27 K Ca Sc Ti V Cr Mn Fe 39. 10 37 40. 08 38 44. 96 39 47. 88 40 50. 94 41 52. 00 42 54. 94 43 55. 85 44 11 12 Na Mg 22. 99 19 9 10 ------- VIII ------- 8 ------- 11 IB 1 B 12 IIB 2 B 13 14 15 16 17 18 Al Si P S Cl Ar 26. 98 28. 09 30. 97 32. 07 35. 45 39. 95 28 29 30 31 32 33 34 35 36 Co Ni Cu Zn Ga Ge As Se Br Kr 58. 47 45 58. 69 46 63. 55 47 65. 39 48 69. 72 49 72. 59 50 74. 92 51 78. 96 52 79. 90 53 83. 80 54 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe 85. 47 55 87. 62 56 88. 91 57 91. 22 72 92. 91 73 95. 94 74 (98) 75 101. 1 76 102. 9 77 106. 4 78 107. 9 79 112. 4 80 114. 8 81 118. 7 82 121. 8 83 127. 6 84 126. 9 85 131. 3 86 Cs Ba La* Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn 132. 9 137. 3 138. 9 178. 5 180. 9 183. 9 186. 2 190. 2 195. 1 197. 0 200. 5 204. 4 207. 2 209. 0 (210) (222) 87 7 13 IIIA 3 A 88 89 109 110 111 112 114 116 118 Fr Ra Ac~ Rf Db Sg Bh Hs Mt --- --- --- (223) (226) (227) (257) 104 (260) 105 (263) 106 (262) 107 (265) 108 (266) () () () 58 Lanthanide Series* Actinide Series~ 59 60 61 62 63 64 65 66 67 68 69 70 71 Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 140. 1 90 140. 9 91 144. 2 92 (147) 93 150. 4 94 152. 0 95 157. 3 96 158. 9 97 162. 5 98 164. 9 99 167. 3 100 168. 9 101 173. 0 102 175. 0 103 Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr 232. 0 (231) (238) (237) (242) (243) (247) (249) (254) (253) (256) (254) (257) Courtesy of Prof. K. Kim, Gyeongsang National University, Korea http: //periodic. lanl. gov

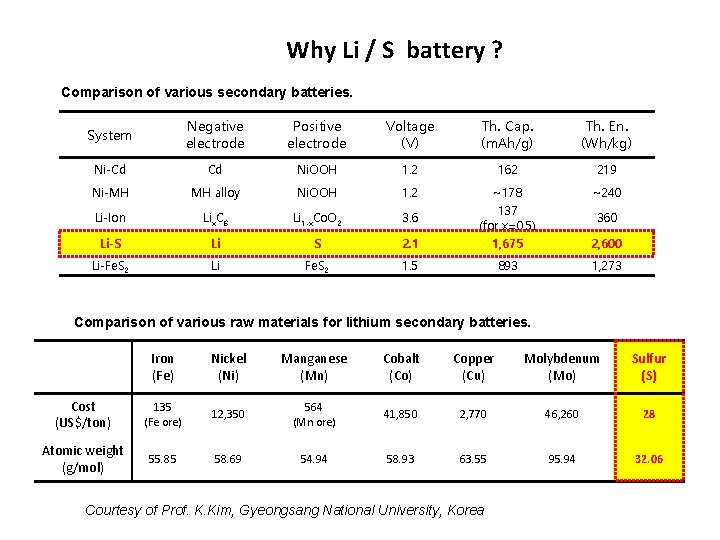
Why Li / S battery ? Comparison of various secondary batteries. System Negative electrode Positive electrode Voltage (V) Th. Cap. (m. Ah/g) Th. En. (Wh/kg) Ni-Cd Cd Ni. OOH 1. 2 162 219 Ni-MH MH alloy Ni. OOH 1. 2 ~178 ~240 Li-Ion Lix. C 6 Li 1 -x. Co. O 2 3. 6 137 (for x=0. 5) 360 Li-S Li S 2. 1 1, 675 2, 600 Li-Fe. S 2 Li Fe. S 2 1. 5 893 1, 273 Comparison of various raw materials for lithium secondary batteries. Iron (Fe) Nickel (Ni) Manganese (Mn) Cobalt (Co) Copper (Cu) Molybdenum (Mo) Sulfur (S) Cost (US$/ton) 135 (Fe ore) 12, 350 564 (Mn ore) 41, 850 2, 770 46, 260 28 Atomic weight (g/mol) 55. 85 58. 69 54. 94 58. 93 63. 55 95. 94 32. 06 Courtesy of Prof. K. Kim, Gyeongsang National University, Korea

The lithium-sulfur battery The Li/S concept is not new. However, so far limited progress due to a series of practical issues Major Issues: solubility of the polysulphides Lix. Sy in the electrolyte (loss of active mass low utilization of the sulphur cathode and in severe capacity decay upon cycling) low electronic conductivity of S , Li 2 S and intermediate Li-S products (low rate capability, isolated active material) Reactivity of the lithium metal anode (dendrite deposition, cell shorting, safety)

R&D is required to improve the performance of super-batteries, such as Li-S or Li-O 2 to meet the HEV-EV requirement Large investments are in progress. worldwide to reach this important goal

Our approach: Total renewal of the battery chemistry, including all three components, i. e. anode, electrolyte and cathode. ANODE Conventional : Li metal our work : Sn-C nanocomposite (gain in reliability and in cycle life) ELECTROLYTE Conventional : liquid organic our work : gel-polymer membrane (gain in safety and cell fabrication) CATHODE Conventional : sulfur-carbon our work : C- Li 2 S composite Conventional : liquid organic (Li-metal-free battery ) (Li metal battery) Jusef Hassoun and Bruno Scrosati, Angew. Chem. Int. Ed. 2010, 49, 2371 http: //www. wiley-vch. de/vch/journals/2002/press/201010 press. html

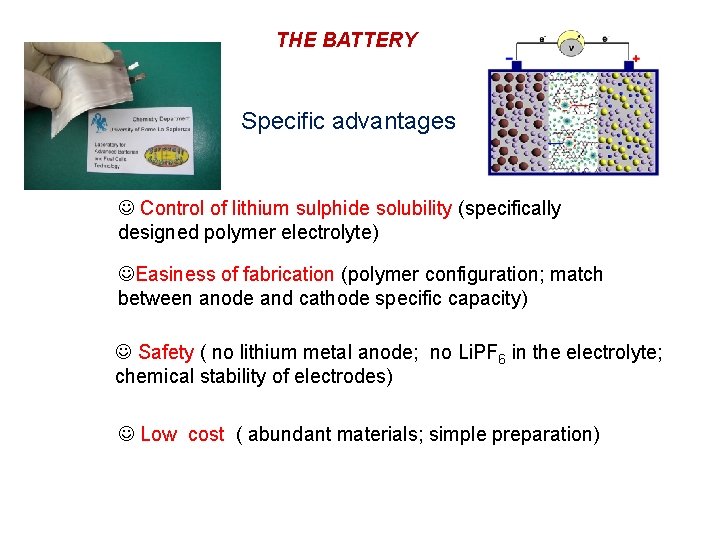
THE BATTERY Specific advantages Control of lithium sulphide solubility (specifically designed polymer electrolyte) Easiness of fabrication (polymer configuration; match between anode and cathode specific capacity) Safety ( no lithium metal anode; no Li. PF 6 in the electrolyte; chemical stability of electrodes) Low cost ( abundant materials; simple preparation)

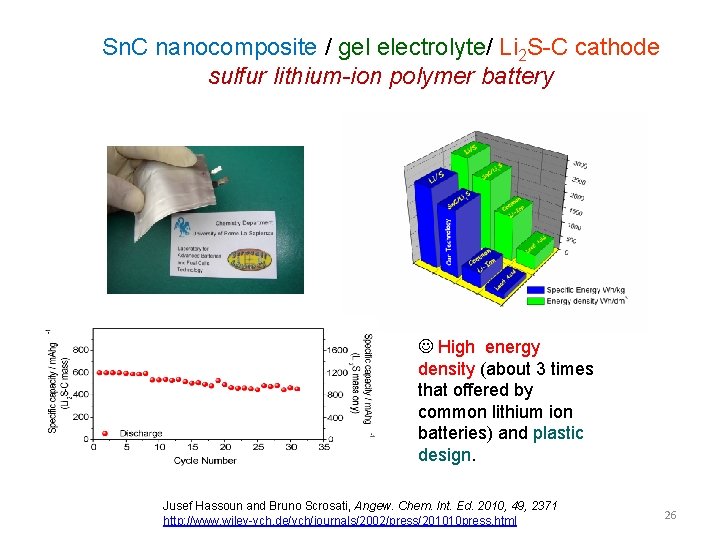
Sn. C nanocomposite / gel electrolyte/ Li 2 S-C cathode sulfur lithium-ion polymer battery High energy density (about 3 times that offered by common lithium ion batteries) and plastic design. Jusef Hassoun and Bruno Scrosati, Angew. Chem. Int. Ed. 2010, 49, 2371 http: //www. wiley-vch. de/vch/journals/2002/press/201010 press. html 26

Acknowledgement Funds Italian Ministry of Education , University and Resarch, MIUR , PRIN 2007 Project and SIID Project “REALIST” (Rechargeable, advanced, nano structured lithium batteries with high energy storage) sponsored by Italian Institute of Technology.
 Traguardi per lo sviluppo delle competenze irc
Traguardi per lo sviluppo delle competenze irc Secondo congedo saba
Secondo congedo saba La gestione per conto dello stato corso sicurezza
La gestione per conto dello stato corso sicurezza Stato ideale per platone
Stato ideale per platone Chimica piu capitolo 19 soluzioni
Chimica piu capitolo 19 soluzioni Studia le ossido riduzioni
Studia le ossido riduzioni Elettrodo seconda specie
Elettrodo seconda specie Cinetica ordine zero
Cinetica ordine zero Decimo quinta estacion via crucis
Decimo quinta estacion via crucis Via erudita e via popular
Via erudita e via popular Via positiva and via negativa
Via positiva and via negativa Santo viacrucis
Santo viacrucis Motoneurona superior e inferior anatomia
Motoneurona superior e inferior anatomia Balance energetico
Balance energetico Sistema energetico
Sistema energetico Cadena respiratoria ciclo de krebs
Cadena respiratoria ciclo de krebs Fabbisogno energetico
Fabbisogno energetico Cadeia respiratoria
Cadeia respiratoria Acetil coa
Acetil coa Fise word
Fise word Fabbisogno energetico
Fabbisogno energetico Fotovoltaico eolico comiter
Fotovoltaico eolico comiter Quoziente energetico formula
Quoziente energetico formula Ciclo de krebs nadh
Ciclo de krebs nadh Génesys ahorrador energético
Génesys ahorrador energético Fabbisogno energetico
Fabbisogno energetico Kcal por kg de peso
Kcal por kg de peso Balanço energetico
Balanço energetico