STATES OF MATTER AND CHANGES OF STATE SOLID









- Slides: 18

STATES OF MATTER AND CHANGES OF STATE

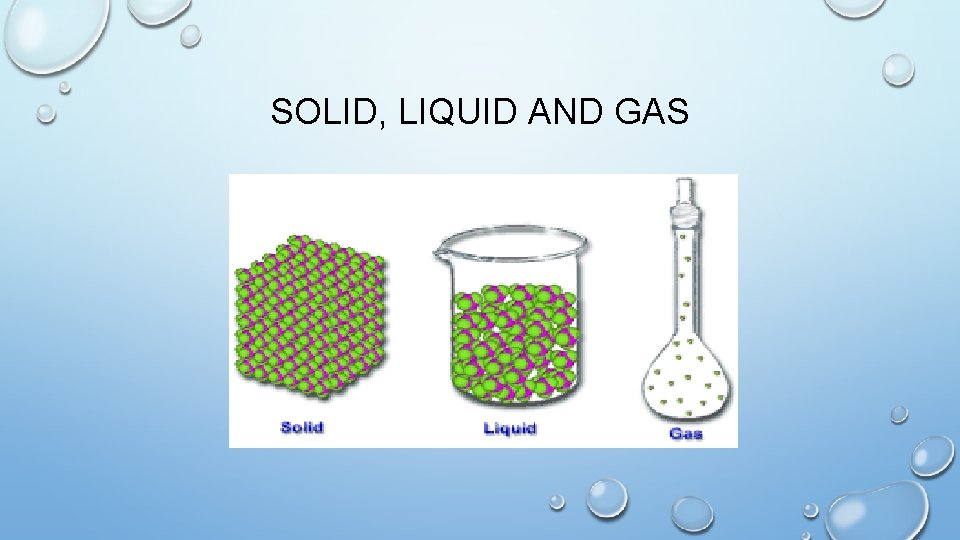
SOLID, LIQUID AND GAS

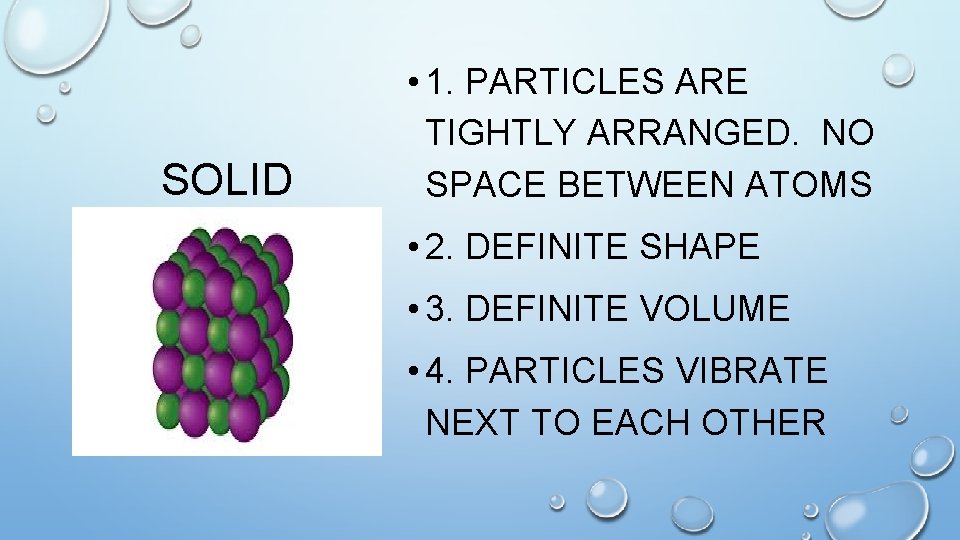
SOLID • 1. PARTICLES ARE TIGHTLY ARRANGED. NO SPACE BETWEEN ATOMS • 2. DEFINITE SHAPE • 3. DEFINITE VOLUME • 4. PARTICLES VIBRATE NEXT TO EACH OTHER

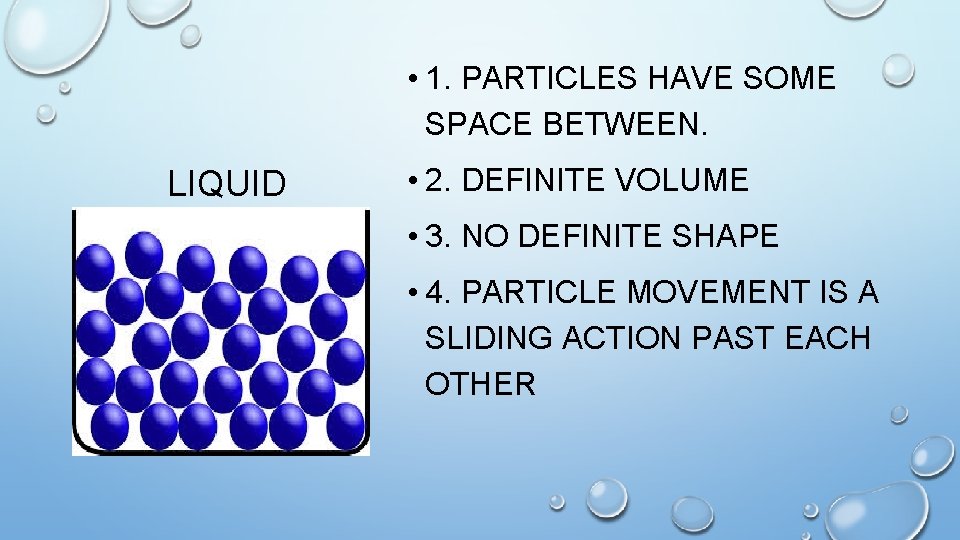
• 1. PARTICLES HAVE SOME SPACE BETWEEN. LIQUID • 2. DEFINITE VOLUME • 3. NO DEFINITE SHAPE • 4. PARTICLE MOVEMENT IS A SLIDING ACTION PAST EACH OTHER

• 1. PARTICLE SPACING IS VERY FAR APART GAS • 2. NO DEFINITE SHAPE • 3. NO DEFINITE VOLUME • 4. PARTICLE MOVEMENT IS VERY FAST AND MAY OR MAY NOT COME IN CONTACT WITH EACH OTHER

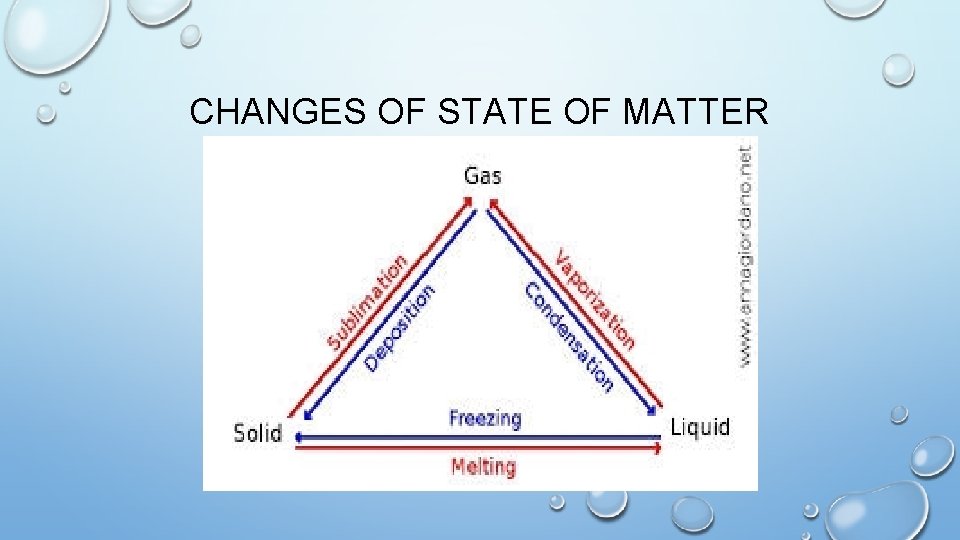
CHANGES OF STATE OF MATTER

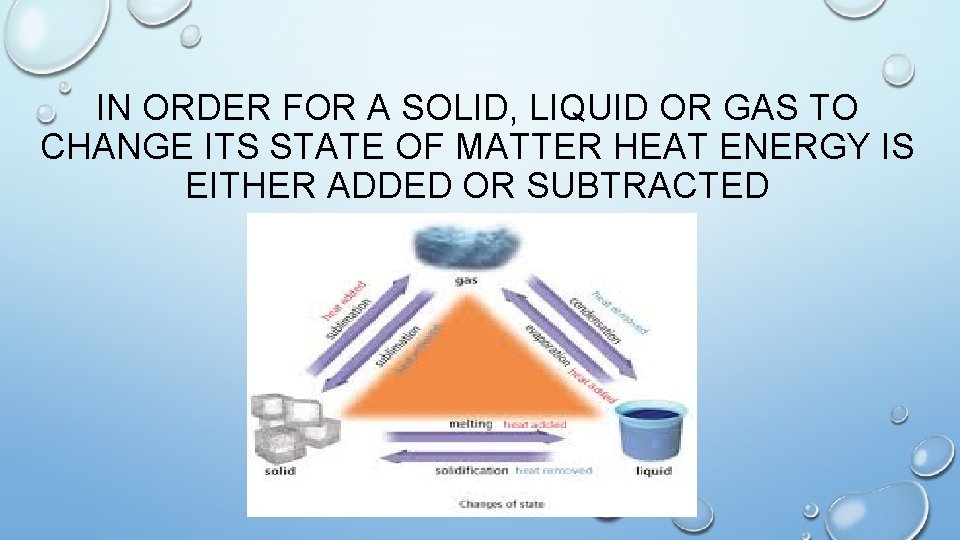
IN ORDER FOR A SOLID, LIQUID OR GAS TO CHANGE ITS STATE OF MATTER HEAT ENERGY IS EITHER ADDED OR SUBTRACTED

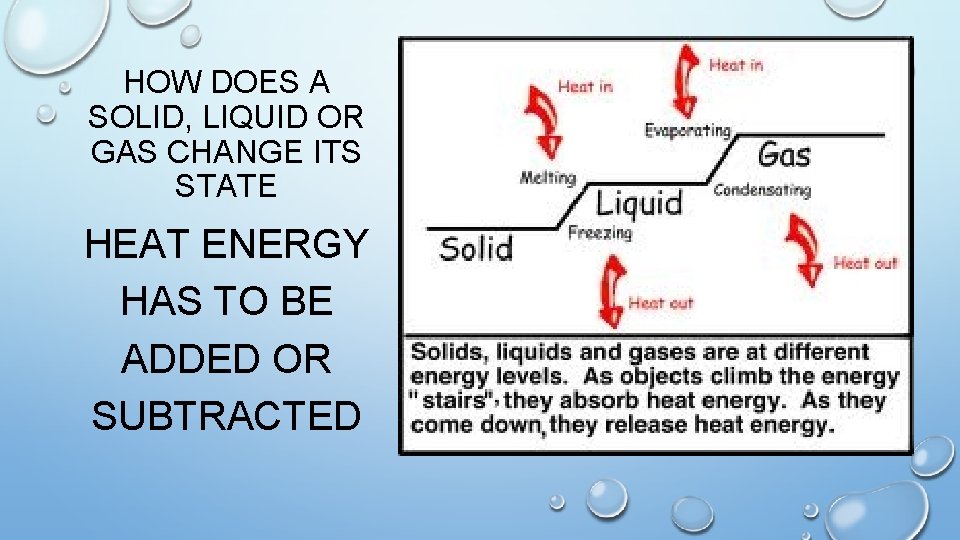
HOW DOES A SOLID, LIQUID OR GAS CHANGE ITS STATE HEAT ENERGY HAS TO BE ADDED OR SUBTRACTED

HEAT ENERGY ADDED TO A SOLID-MELTING OCCURS 97˚F/16˚C KITCHEN MELTING POINTS MELTED GOLD

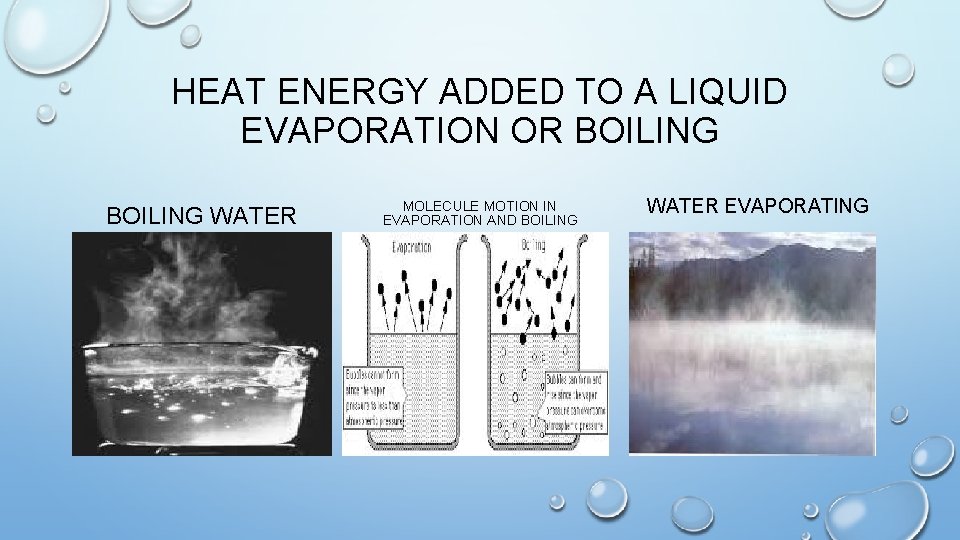
HEAT ENERGY ADDED TO A LIQUID EVAPORATION OR BOILING WATER MOLECULE MOTION IN EVAPORATION AND BOILING WATER EVAPORATING

COMPARISON OF BOILING AND EVAPORATION

SUBLIMATION A SOLID CHANGES INTO A GAS WITHOUT BECOMING A LIQUID FIRST DRY ICE SNOW SUBLIMATION DRY ICE

WHEN HEAT ENERGY IS REMOVED A GAS BECOMES A LIQUID CONDENSATION WATER VAPOR CONDENSING ON SODA LITER WATER VAPOR CONDENSING ON GLASS WATER VAPOR CONDENSING ON PLANTS

REMOVAL OF HEAT ENERGY FROM A LIQUID FREEZING THERE IS A SPECIFIC TEMP AT WHICH LIQUIDS FREEZE

WATER FREEZING ALMOST IMMEDIATELY

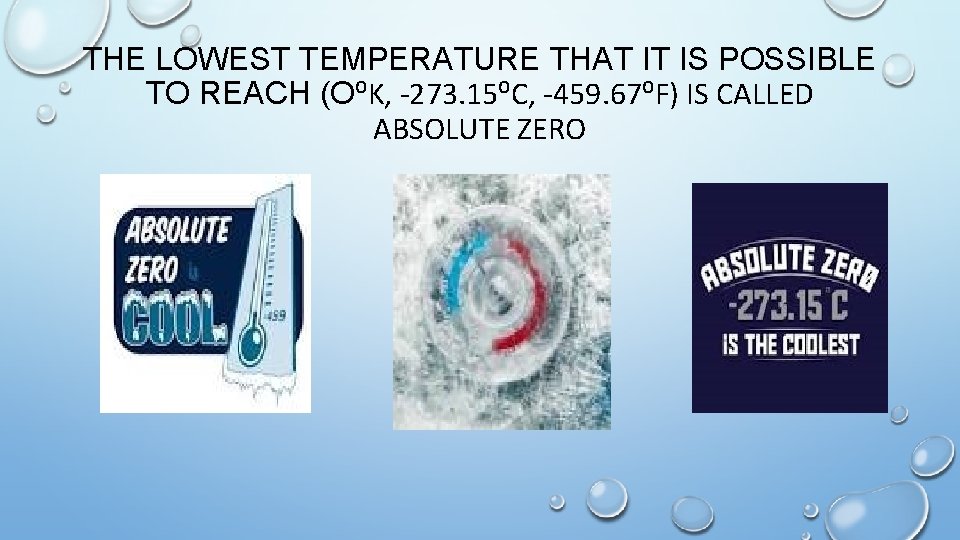
THE LOWEST TEMPERATURE THAT IT IS POSSIBLE TO REACH (O⁰K, -273. 15⁰C, -459. 67⁰F) IS CALLED ABSOLUTE ZERO

DEPOSITION WHEN HEAT ENERGY IS REMOVED AND WATER VAPOR CHANGES DIRECTLY INTO A SOLID FROST SNOW FROST

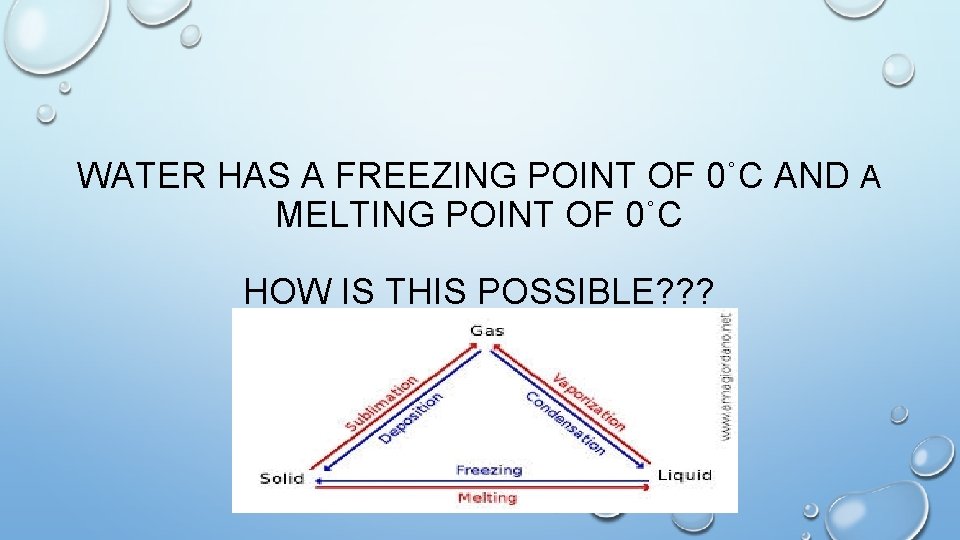
WATER HAS A FREEZING POINT OF 0˚C AND A MELTING POINT OF 0˚C HOW IS THIS POSSIBLE? ? ?
 Solid
Solid States of matter solid liquid gas
States of matter solid liquid gas Change of state of matter
Change of state of matter Elizabeth mulroney
Elizabeth mulroney What is chemical change
What is chemical change Properties and changes of matter worksheet
Properties and changes of matter worksheet Chemistry matter and its changes
Chemistry matter and its changes Matter-properties and changes answer key
Matter-properties and changes answer key Properties of and changes in matter grade 5
Properties of and changes in matter grade 5 Eating food physical or chemical change
Eating food physical or chemical change Chem
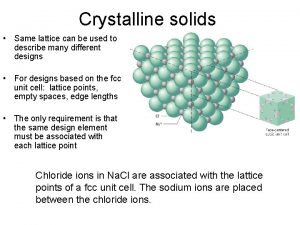
Chem Crystalline solid and amorphous solid
Crystalline solid and amorphous solid Crystalline solid
Crystalline solid Crystalline solid and amorphous solid
Crystalline solid and amorphous solid Crystalline solid and amorphous solid
Crystalline solid and amorphous solid Concept map of states of matter
Concept map of states of matter Physical property examples
Physical property examples Phases changes of matter
Phases changes of matter Two types of changes in matter
Two types of changes in matter