Solutions Chapter 12 Types of solutions Suspensions largest
























- Slides: 58

Solutions Chapter 12

Types of solutions • Suspensions – largest particle size – > 1000 nm – Blood, paint, aerosols, muddy water • Colloids – mid sized particles – 2 -1000 nm – Milk, fog, butter • Solution – smallest particles – <2 nm – Air, seawater, gasoline

Types of colloids Aerosol – liquid in gas Solid Aerosol – solid in gas Sol -- solid in liquid like protein particles in milk

Emulsion – liquid in liquid like oil droplets in mayonnaise. Foams – gases in liquids like whipped cream Solid emulsion – liquid in a solid like milk in butter Gel – a solid emulsion which is soft but holds its shape like Jell-O

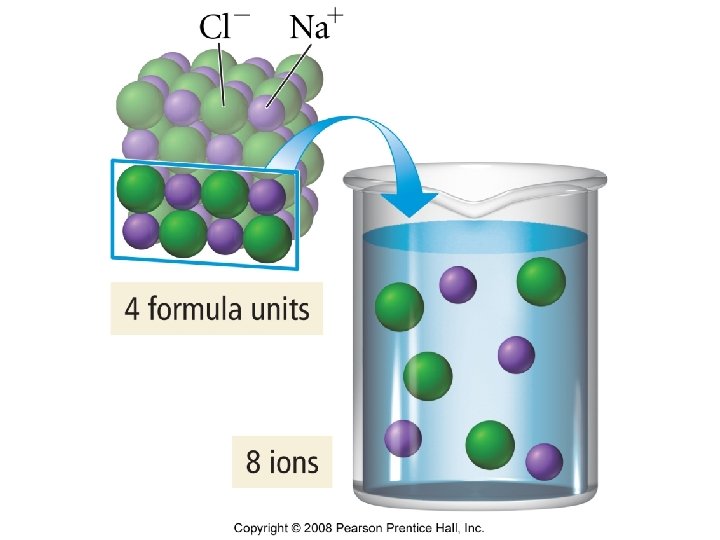
Key terms • Solution - A general term for a solute dissolved in a solvent. A homogeneous mixture of 2 or more components in which particles intermingle at the molecular level. • Solvent - The component of a solution that is the greater quantity. • Solute - The component of a solution that is the lesser quantity.

• • Solution Types Gas in gas Gas in liquid Gas in solid Liquid in liquid Miscible - refers to 2 or more liquids that are infinitely soluble in one another. Immiscible - refers to 2 liquids that are not soluble in one another and if mixed separate into 2 layers. • Liquid in solid • Solid in liquid • Solid in solid

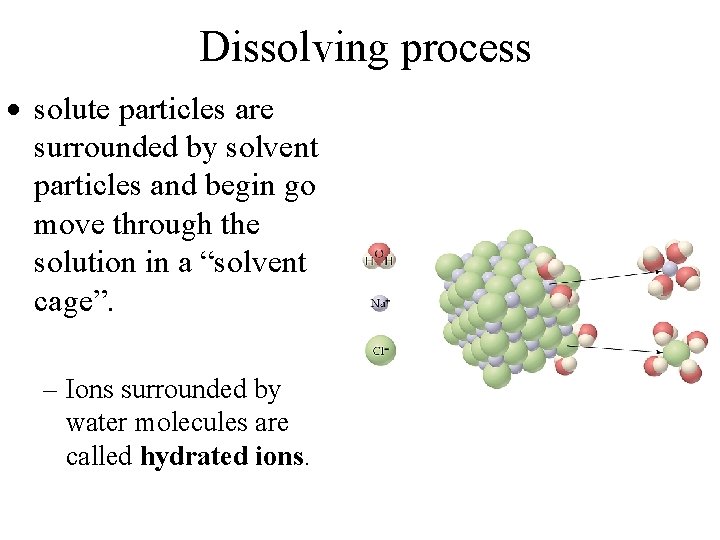
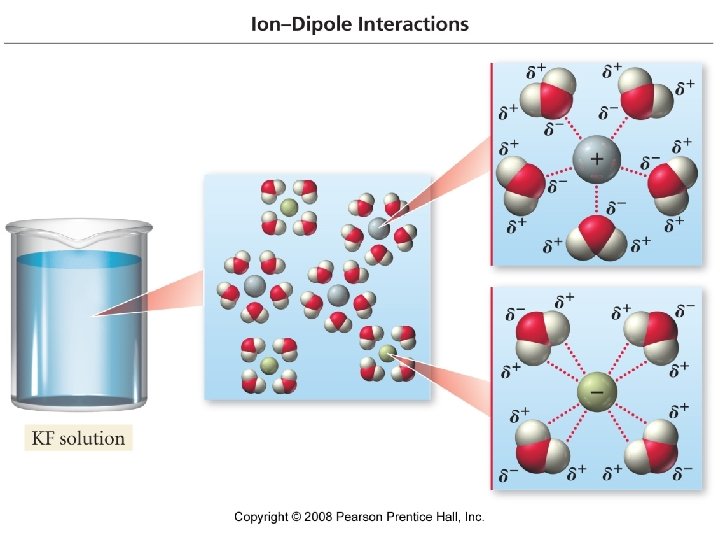
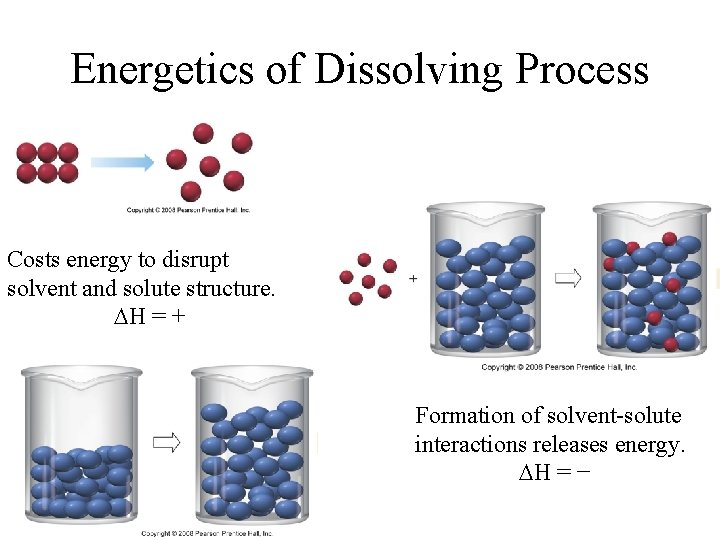
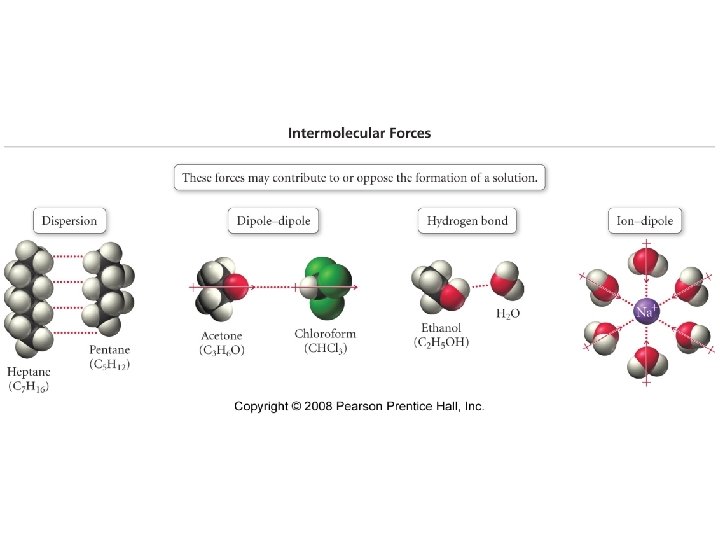
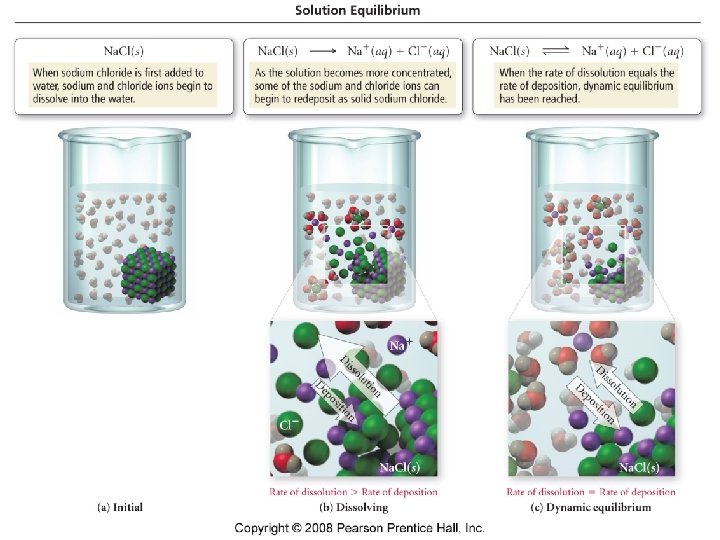
Dissolving process solute particles are surrounded by solvent particles and begin go move through the solution in a “solvent cage”. – Ions surrounded by water molecules are called hydrated ions.


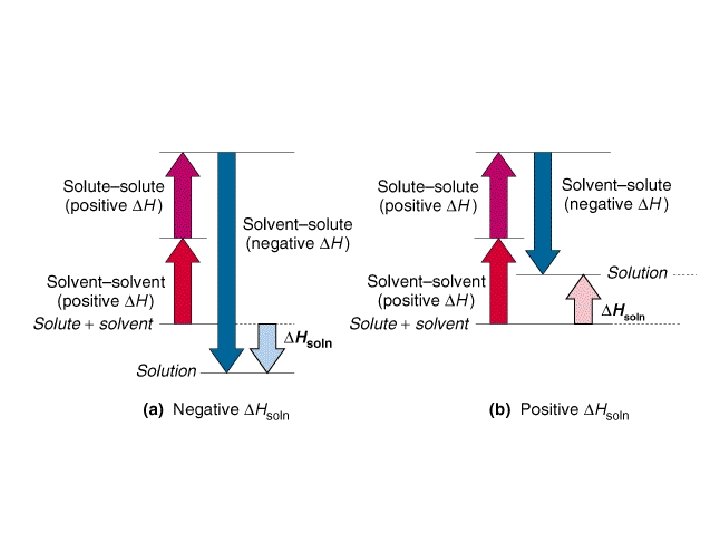
Energetics of Dissolving Process • G = H - T S • H can be either + or -, it depends on – the enthalpy to break the crystal apart – the enthalpy of disrupting solvent structure – the enthalpy change for hydrating solute. • S is generally positive.

Energetics of Dissolving Process Costs energy to disrupt solvent and solute structure. H = + Formation of solvent-solute interactions releases energy. H = −



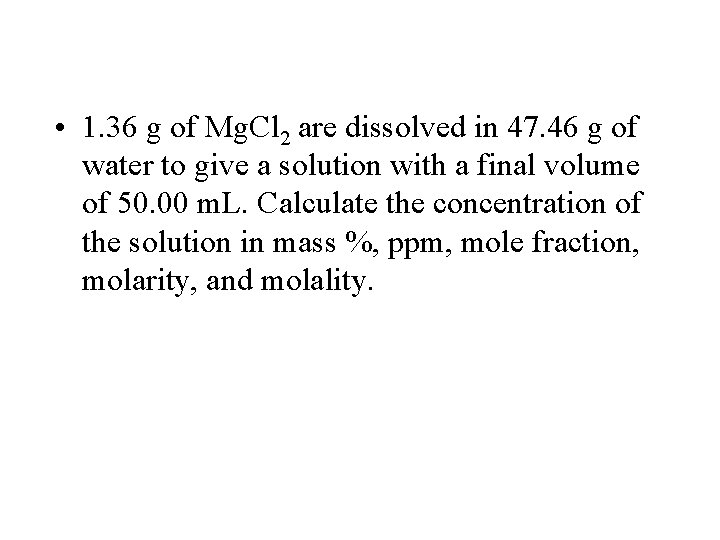
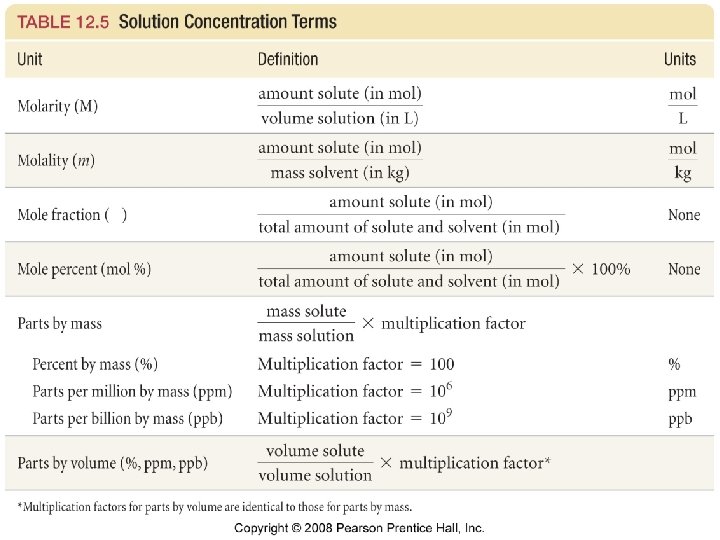
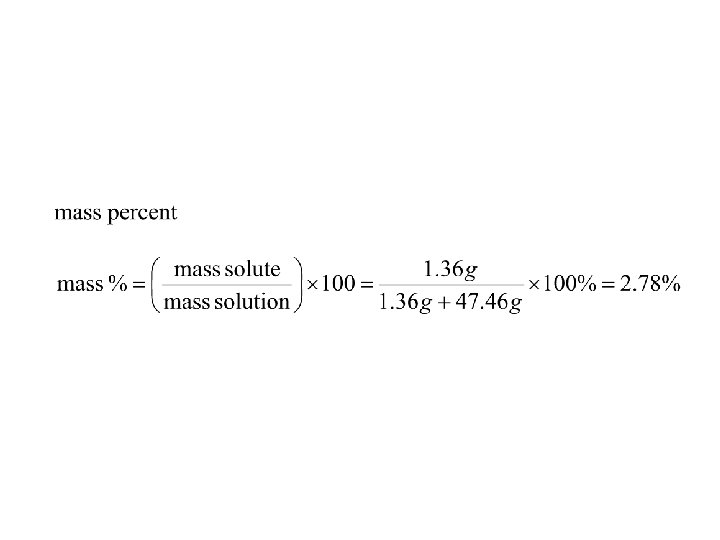
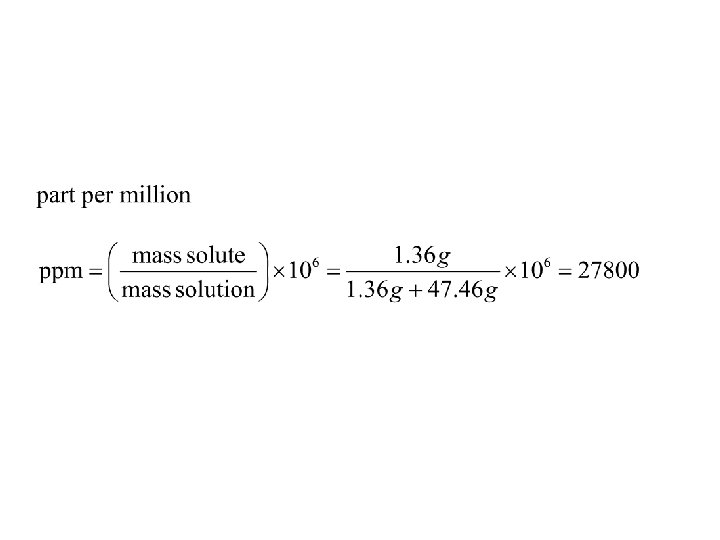
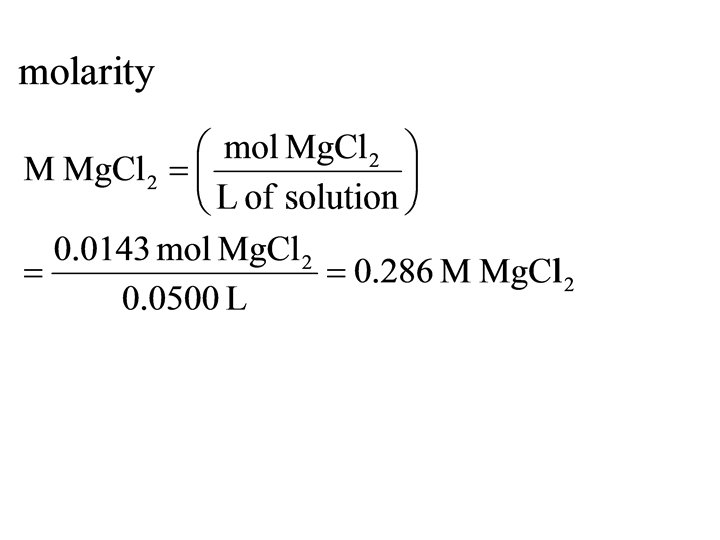
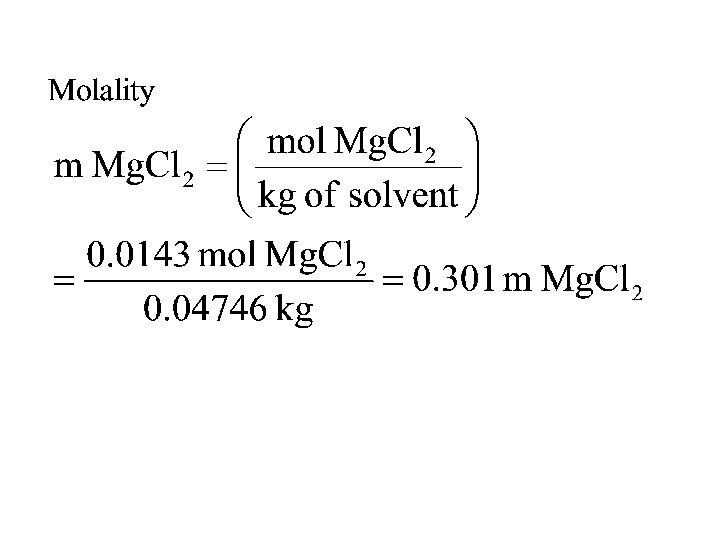
• 1. 36 g of Mg. Cl 2 are dissolved in 47. 46 g of water to give a solution with a final volume of 50. 00 m. L. Calculate the concentration of the solution in mass %, ppm, mole fraction, molarity, and molality.






• Hydrochloric acid is sold as a concentrated aqueous solution. The concentration of commercial HCl is 11. 7 M and its density is 1. 18 g/m. L. Calculate the mass percent of HCl in the solution. Calculate the molality of the solution.

• Concentrated sulfuric acid has a density of 1. 84 g/m. L and is 18 M. What is the mass % H 2 SO 4 in the solution? What is the molality of H 2 SO 4 in the solution?


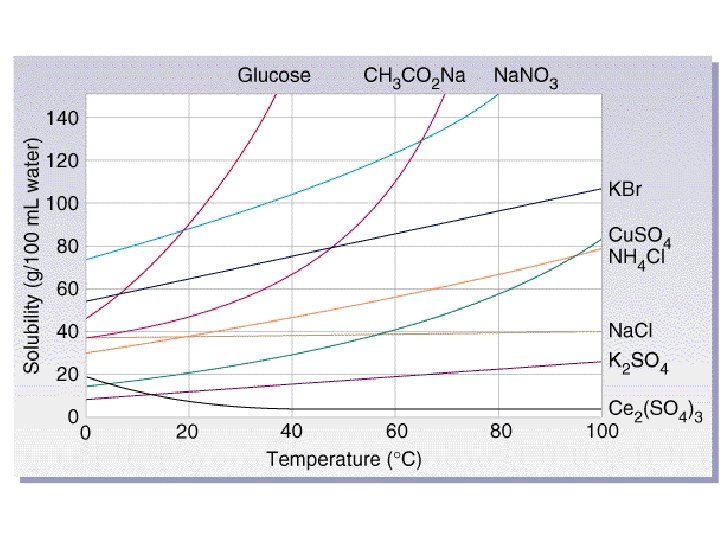
Solubility - the maximum amount of solute than can dissolve in 100 g of solvent at a given temperature. • Saturated solution - a solution that contains the maximum amount of dissolved solute that will dissolve at a given temperature. Unsaturated solution - a solution that contains less solute than can be dissolved at a given temperature. • Supersaturated solution - a solution that contains more dissolved solute than will ordinarily dissolve at a given temperature.

Factors Affecting Solubility

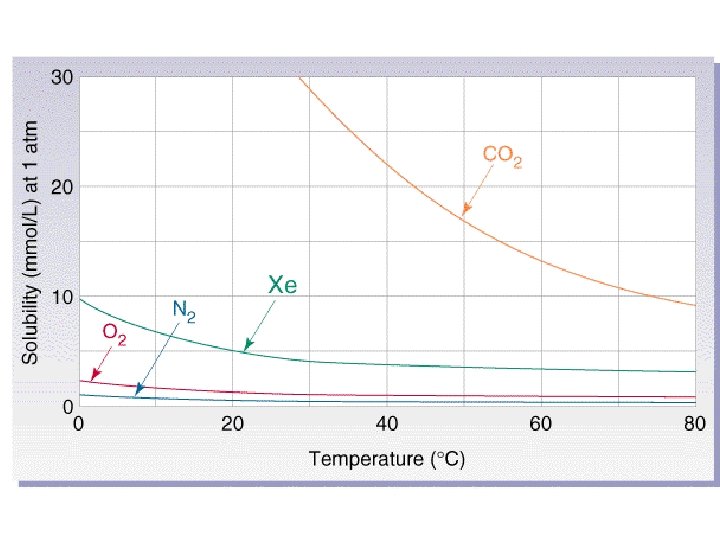
Temperature • For gases, solubility decreases as temperature increases. • For liquids and solids solubility generally increases as temperature increases.



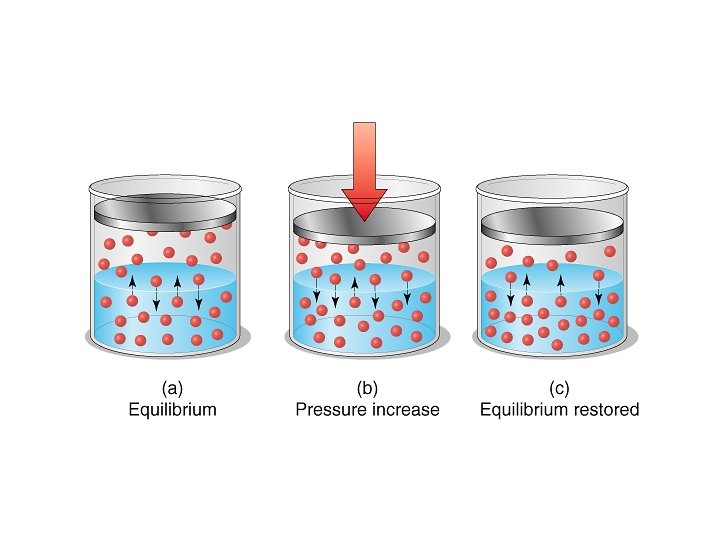
Pressure • Pressure has very little effect on the solubility of liquids and solids. • Pressure is very important to the solubility of gases however.


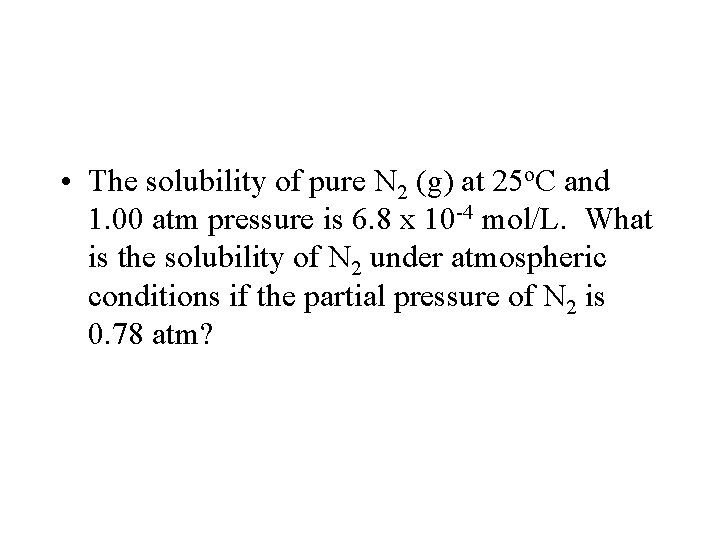
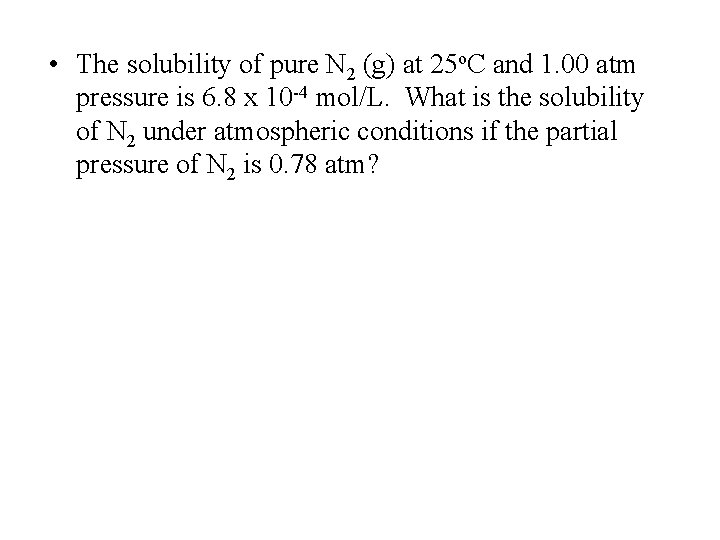
Henry’s Law • The solubility of a gas is directly proportional to its partial pressure above the solution. • Or Solubility = k. P – where k = henry’s law constant

• The solubility of pure N 2 (g) at 25 o. C and 1. 00 atm pressure is 6. 8 x 10 -4 mol/L. What is the solubility of N 2 under atmospheric conditions if the partial pressure of N 2 is 0. 78 atm?

• The solubility of pure N 2 (g) at 25 o. C and 1. 00 atm pressure is 6. 8 x 10 -4 mol/L. What is the solubility of N 2 under atmospheric conditions if the partial pressure of N 2 is 0. 78 atm?

Colligative Properties


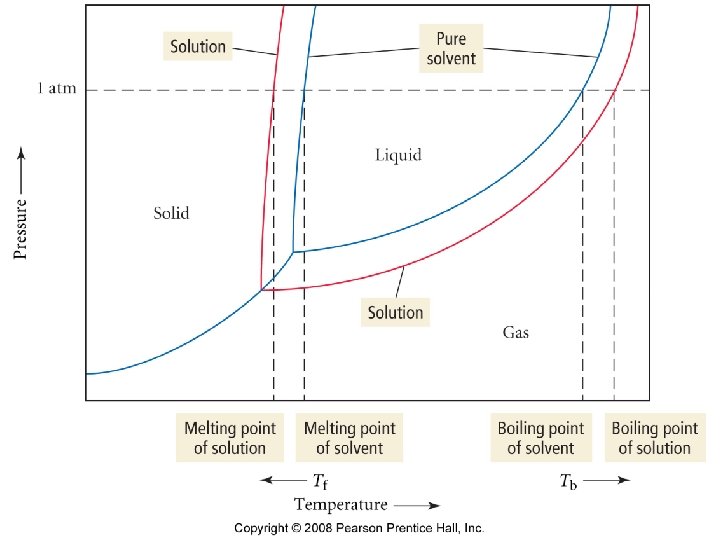
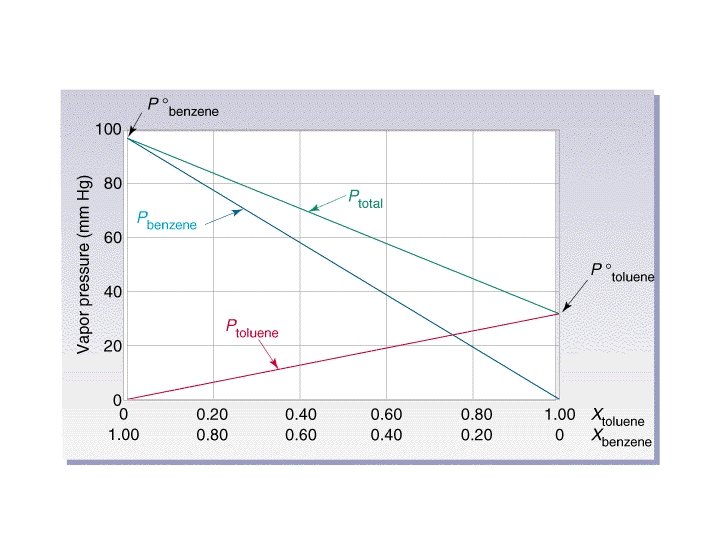
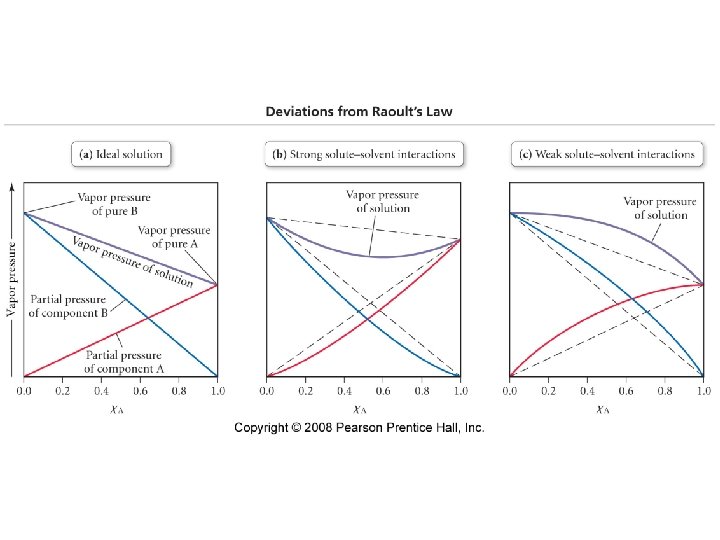
Vapor pressure lowering • The vapor pressure of a solution is always lower than the vapor pressure of the corresponding pure solvent. • Raoult’s Law • P solution = (P solvent )(X solvent)

• What is the vapor pressure of a solution made of 10. 0 g of glucose and 100. 0 g water at 37. 0 o. C? (Vapor pressure of water at 37 o. C is 47. 1 torr. )


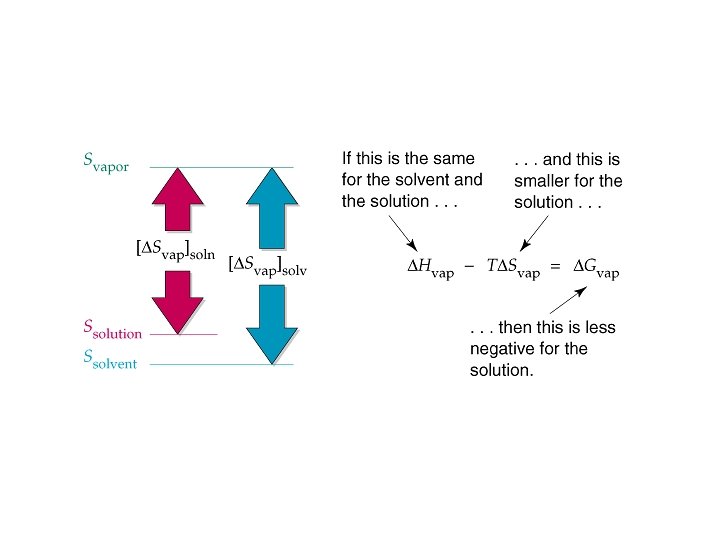
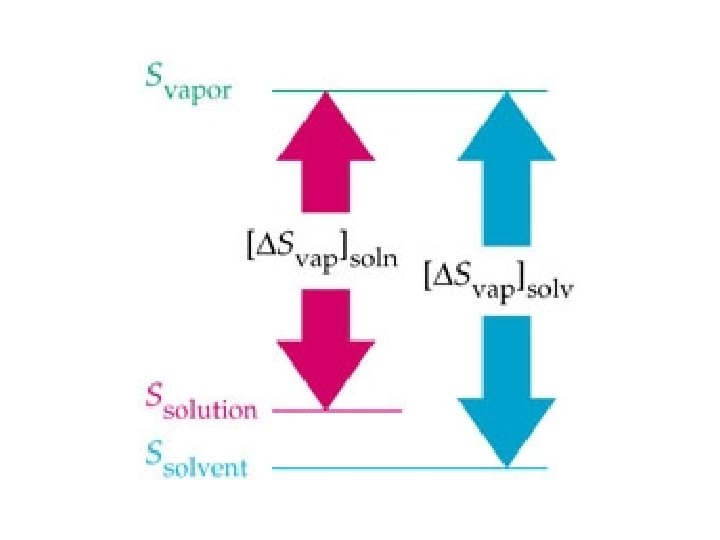
Why is vapor pressure lowered in solutions? • Gvap = Hvap - T Svap


Solutions with a volatile solute • P total = PA + PB • PA = (XA)(PAo) • PB = (XB)(PBo) – PAo is the vapor pressure of the pure solvent.


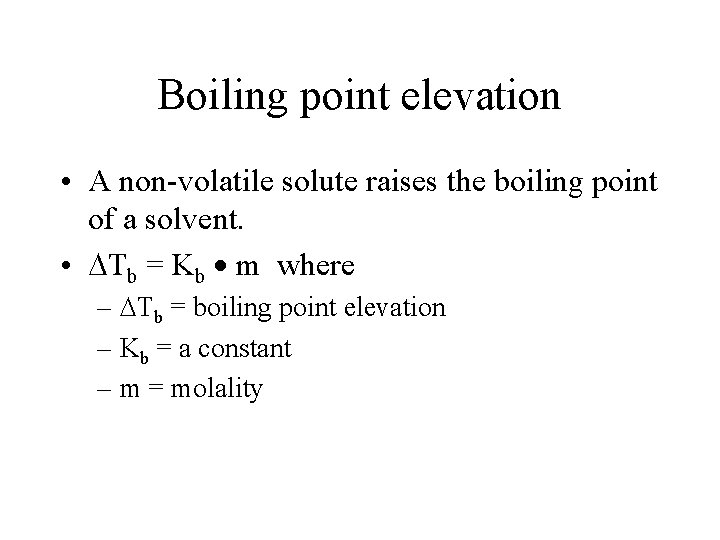
Boiling point elevation • A non-volatile solute raises the boiling point of a solvent. • Tb = Kb m where – Tb = boiling point elevation – Kb = a constant – m = molality

Freezing point depression • A non-volatile solute depresses the freezing point of a solvent. • Tf = Kf m where – Tf = freezing point depression – Kf = a constant – m = molality


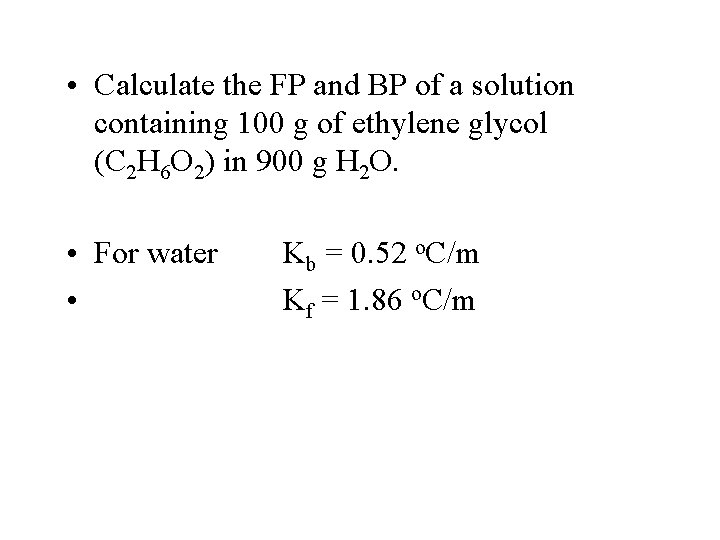
• Calculate the FP and BP of a solution containing 100 g of ethylene glycol (C 2 H 6 O 2) in 900 g H 2 O. • For water • Kb = 0. 52 o. C/m Kf = 1. 86 o. C/m

• Tartaric acid can be produced from crystalline residues found in wine vats. It is used in baking powders and as an additive in foods. Analysis show that it is 32. 3%C, 3. 97% H, and the remainder O. When 1. 161 g tartaric acid is dissolved in 11. 23 g water, the solution freezes at – 1. 26 o. C. Determine the empirical and molecular formula for tartaric acid.

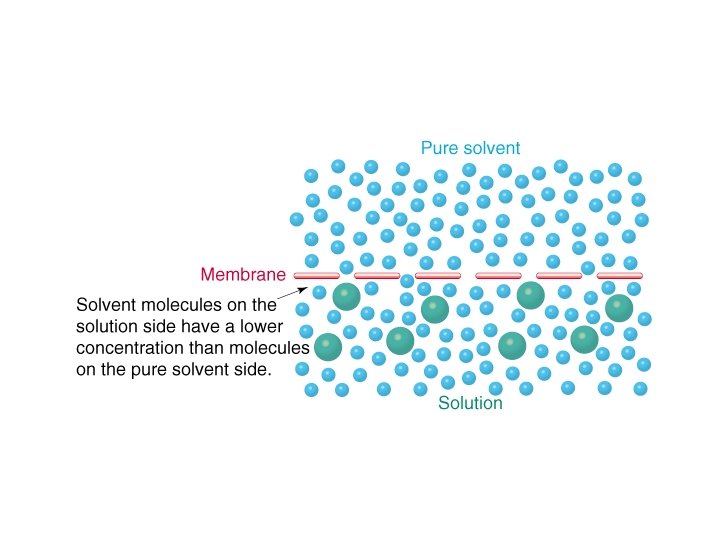
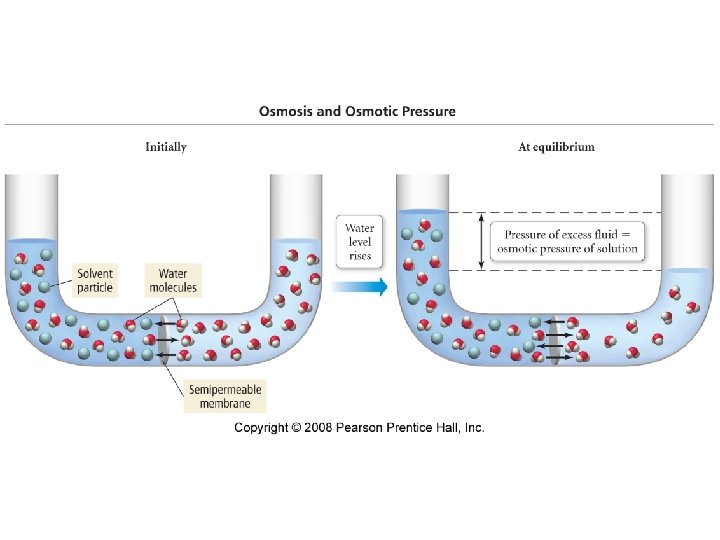
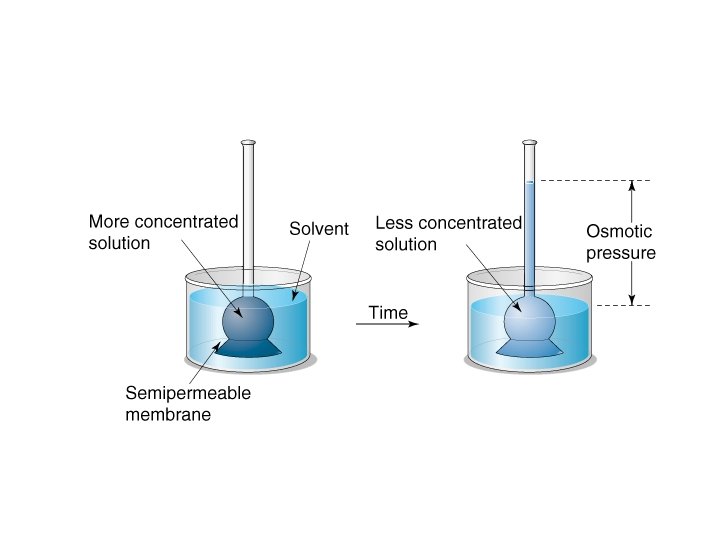
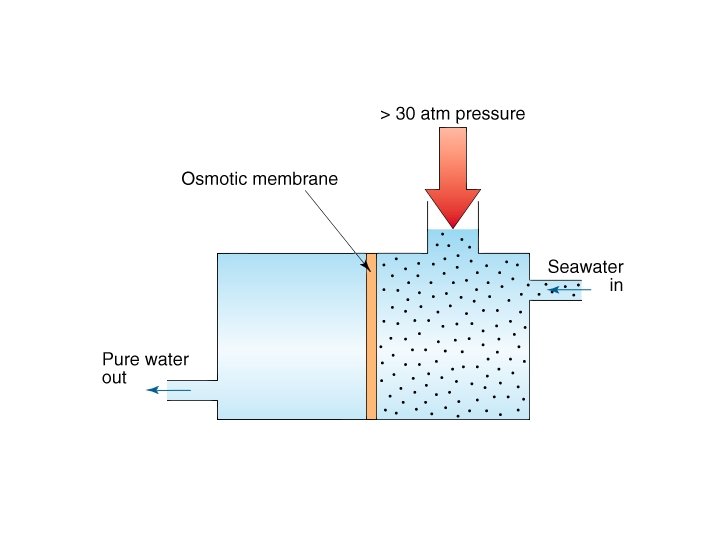
Osmotic Pressure




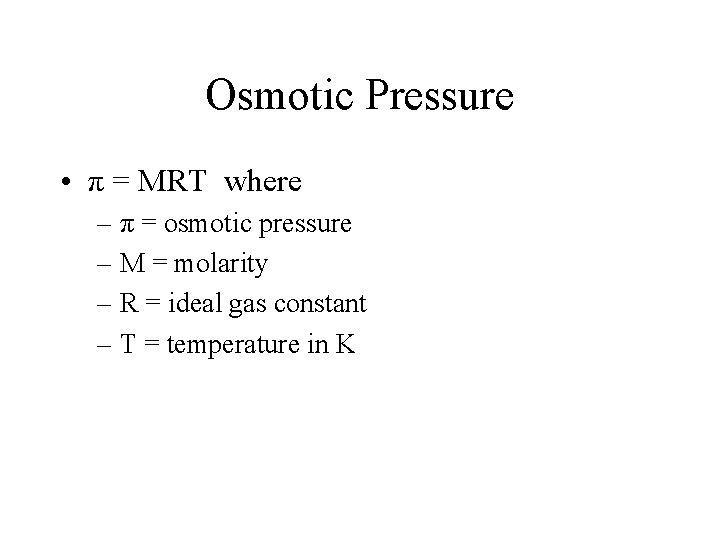
Osmotic Pressure • π = MRT where – π = osmotic pressure – M = molarity – R = ideal gas constant – T = temperature in K

• For a solution containing 3. 00 g of pepsin in 10. 0 m. L of solution π = 0. 213 atm at 25 o. C. What is the molecular mass of pepsin?



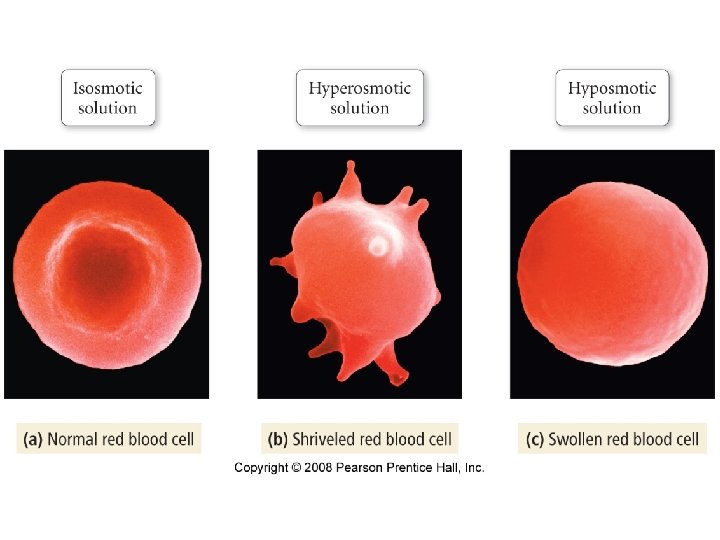
Colligative properties of electrolytes • Colligative properties depend on the number of particles in solution. • Van’t Hoff factor


New equations • Tb = i. Kb m • Tf = i. Kf m • π = i. MRT

• The freezing point depression constants for the solvents cyclohexane and naphthalene are 20. 1 o. C/m and 6. 94 o. C/m respectively. Which would give a more accurate determination by freezing point depression of the molar mass of a substance that is soluble in either solvent? Why?