Solutions Suspensions Colloids Solutions n n n Appears














- Slides: 15

Solutions, Suspensions Colloids

Solutions n n n Appears to be a single substance but really two or more substances dissolved in a solvent and evenly distributed Very small particles that never settle out Homogeneous

Examples of Solutions n n Alloys – solid solutions of metals or nonmetals dissolved in metals Iced tea, salt water, soda, gasoline


Solute versus Solvent n n n A solute is the substance in a solution that gets dissolved A solvent is the substance in a solution that does the dissolving If something is soluble, the solute can be dissolved in a particular solvent If something is insoluble, the solute will not be dissolved in a particular solvent Solutes can be soluble in some solvents but insoluble in others

Concentration n The comparison of solute to solvent When there is not a lot of solute compared to solvent, the solution is dilute When there is a lot of solute compared to solvent, the solution is concentrated

Saturated Solutions n Saturated solution – contains all of the solute it can hold at a given temperature. n If you add even one more molecule of the solute, it will fall out of solution and rest on the bottom

Unsaturated Solutions n Contains less solute than it can hold at a given temperature n Can add more solute and it wont fall out of the solution

Supersaturated Solution n Solution that holds more than it usually would at a given temperature

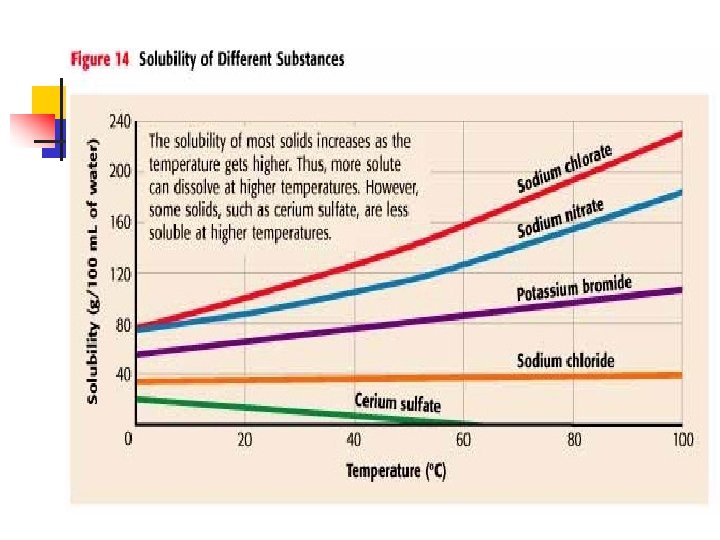
Solubility n n n The amount of solute needed to make a saturated solution in a given solvent at a given temperature For solid solute in liquid solvent – solubility rises as temperature rises. For gas solute in a liquid solvent – solubility lowers as temperature rises ex – soda goes flat at warm temperatures


Methods to speed up dissolving n n n Crushing a solute increases the surface area of the solute allowing more solvent to surround it thus dissolving it faster n Ex. Putting butter in macaroni and cheese Heating a solution increases the energy of the molecules making them move more quickly thus spreading the solute throughout the solvent and speeding up the rate at which the solute dissolves n Ex- cooking iced tea Mixing has the same effect as heating does n Ex- making chocolate milk


Suspensions n n A suspension is a mixture in which particles of material are dispersed throughout a liquid or gas and are large enough to settle out The particles are insoluble Heterogeneous mixtures Ex snow globe

Colloids n n n Have properties of both solutions and suspensions Particles in a colloid are much smaller than particles in a suspension Colloids have the same definition as suspensions in that a colloid is a mixture in which particles of material are dispersed throughout a liquid or gas, but the particles of a colloid are not large enough to settle out Particles in a colloid scatter light Ex milk, fog, jello