Chemistry Thursday 8182016 HOMEWORK 1 1 reading notes





























































![[Property] Neutron Proton Electron Charge 0 +1 -1 Nucleus (center of atom) electron cloud, [Property] Neutron Proton Electron Charge 0 +1 -1 Nucleus (center of atom) electron cloud,](https://slidetodoc.com/presentation_image/53ab5db144cb996b09fa006ea409256d/image-77.jpg)






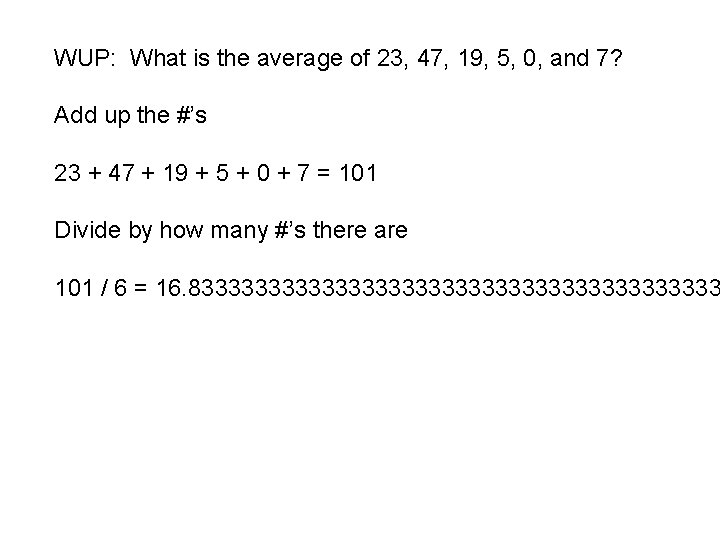






















- Slides: 112

Chemistry Thursday 8/18/2016 HOMEWORK: 1. 1 reading notes, section assessment; 1. 2 reading notes, section assessment. Self-correct using power point in student vault. hhscougars. org Search for Barnes or Daniel Barnes Go to my “Student Vault” page Username = barnesstudent Password = teachamantofish WARM-UP: Work on homework. STAMPS: none today – tonight’s homework will be stamped tomorrow

Chemistry Friday 8/19/2016 HOMEWORK: 1. 3 reading notes WARM-UP: Get out a brand new, blank piece of paper. Name, period, date in upper right-hand corner Title = “ 5 -Week Warm-Up Packet” Skip a line. Write today’s date. Copy the following question: “How have chemists helped tomato farmers fight pinworms? ” Answer the question with one or more complete sentences. STAMPS: 1. 1 reading notes, 1. 1 section assessment, 1. 2 reading notes, 1. 2 section assessment

Student Chemistry On-Line Textbook Log-In Got to powerschool and log in there Click the button at the bottom “Pearson Courses” List of classes pops up Click the book for your chemistry class FORGET IT! I have a pdf on my home page : )

Chemistry Monday 8/22/2016 HOMEWORK: 1. 3 section assessment WARM-UP: Skip a line after your last warm-up. Write today’s date. On the next line, copy the following question: “If you feed different foods to different rats to see how they affect body weight, what do you call the kind of food and what do you call the resulting body weight? ” Answer with one or more complete sentences. STAMPS: 1. 1 notes/sxn assmt; 1. 2 notes/sxn assmt; 1. 3 reading notes

On-Line Textbook Tips 1. Use Firefox or Chrome (NOT Internet Explorer) - Update your browser to the latest version 2. Enable Pop-Ups in your web browser 3. Download or update your Adobe Flash Player to the latest version 4. Download or update Shockwave to the latest version 5. Restart your computer after making any of these changes before trying to access the books

Chemistry Tuesday 8/23/2016 HOMEWORK: Study 1. 1, 1. 2, and especially 1. 3 for tomorrow’s ch 1 quiz. WARM-UP: How did Antoine Lavoisier die? Why? STAMPS: 1. 3 section assessment Done with the warm-up? Get started on the homework.

Chemistry Wednesday 8/24/2016 HOMEWORK: Gather your ch 1 bookwork and lecture notes. They will be turned in tomorrow as a stapled packet. WARM-UP: What makes a hypothesis a good hypothesis? STAMPS: none (test today) Done with the warm-up? Study for the test.

Tests in Mr. Barnes’ Class * Multiple choice is 50% of your grade in the class. It is your top priority. * Don’t panic if you can’t answer a question. Just do your best. * Mr. Barnes grades on a curve. (More on this later. ) * When the buzzer rings, at least guess. 25% > 0%. * If you finish MC section early, answer the screen questions. * Do not copy screen questions. Just put q# & answer. * Still have time left? Get a science magazine Read one or more articles On your scratch paper, write whatever comes to mind Do not interact with your neighbors during a test, even if you’re “done”

Do NOT write on the test packets! (If you want to write something, write it on your scratch paper. )

“Form” = Version Your form is indicated by your red test packet #. Your red test packet # should be the same as your seat #. If your test # is this. . . then your form # is this: 1, 6, 11, 16, 21, 26, 31, 36 = Form 1 2, 7, 12, 17, 22, 27, 32, 37 = Form 2 3, 8, 13, 18, 23, 28, 33, 38 = Form 3 4, 9, 14, 19, 24, 29, 34, 39 = Form 4 5, 10, 15, 20, 25, 30, 35, 40 = Form 5

Scientific Method Quiz Screen Questions 8/24/2016 DIRECTIONS: Answer the following on your scratch paper if and when you finish working on the multiple choice questions. 1. Vernon claims that he’s created an engine oil additive that keeps cars from crashing. He announces that he’s been using the additive for a year and hasn’t crashed yet. What do you have to say to Vernon? 2. How many manipulated variables should an experiment have? Come up with an example that illustrates why. Do NOT involve rats. 3. Compare and contrast hypotheses, theories, and laws. 4. Compare and contrast science and religion.

Chemistry Thursday 8/25/2016 WARNING! Do NOT fill out the safety contract until Mr. Barnes gives you instructions on how to do it! HOMEWORK: Get a parent to sign your safety contract. WARM-UP: What is the most important lab safety rule? STAMPS: none (ch 01 packet submitted today) Done with the warm-up? Read the safety contract. (Get it from the front table if you don’t have it already. )

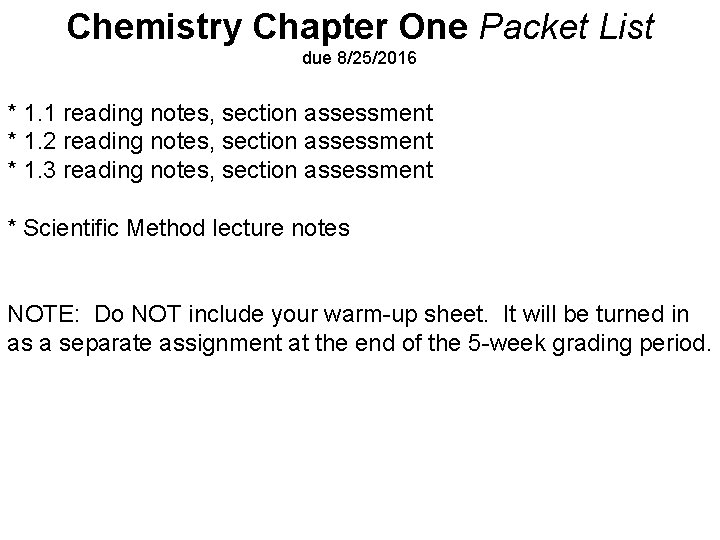
Chemistry Chapter One Packet List due 8/25/2016 * 1. 1 reading notes, section assessment * 1. 2 reading notes, section assessment * 1. 3 reading notes, section assessment * Scientific Method lecture notes NOTE: Do NOT include your warm-up sheet. It will be turned in as a separate assignment at the end of the 5 -week grading period.

Student Safety Contract Instructions * Blue or black ink only. This is a legal document. NO PENCIL * Get it right the first time. No changes = no cross-outs, no whiteouts, no erasures (can’t use pencil anyway). * Parents must date their own signature. Do NOT write the date for your parent’s signature, or the whole contract is disqualified. * Print your first & last name neatly in the blank in the ULHC of the backside. * On the allergy question, say what you are allergic to. * Also list if you have any other respiratory weaknesses in the allergy blanks (asthma, etc. ).

Safety Quiz Instructions * Blue or black ink only. This is a legal document. NO PENCIL * You can make mistakes at first, but, as we go over the quiz, you must correct your mistakes. * Any time you change an answer, you must write your initials next to the correction. * You are responsible for making sure that every answer on your safety quiz is correct.

Chemistry Friday 8/26/2016 HOMEWORK: Get a parent to sign your safety contract if you haven’t done so already (hard due date next Friday). WARM-UP: How do you clean up broken glass? STAMPS: none Done with the warm-up? Read 2. 1 & take notes. (It’ll be due pretty soon. )

RE-TAKES in BARNES’ CLASS * WHEN: During lunch or after school. Bring food if you want to. * EFFECT: If you score higher than your previous score, the new score replaces the old score. If you score less than or equal to, no effect. You risk only your time. No worries. * ELIGIBILITY: You must prove that you’ve done the bookwork for all textbook sections covered by the test in question. Bring your bookwork to get your answer document. * MC/SCR: You can do either the multiple choice or the scratch paper or both. Each has its own separate chance of replacing your previous score in that item. * AGAIN? If you want to re-take a re-take, you’ll need proof of preparation beyond the book (tutoring, Quiz Show, etc. ).

Chemistry Monday 8/29/2016 HOMEWORK: 2. 1 reading notes WARM-UP: How is mercury different from other metals? STAMPS: none Done with the warm-up? Get started on the homework.

Chapter 01 Packet Grading Name, period, and date in upper right hand corner of each sheet of paper, or no points for anything on that sheet. NPD (1) prevents theft and (2) allows return of separated papers. Unstamped work gets MUCH less points than stamped work. (1 point/4 full pages) Packets can be submitted late and/or re-submitted with no penalty. (Not getting stamps is the only lateness penalty. ) Any questions? Any funny red marks you need translated?

Chemistry Tuesday 8/30/2016 HOMEWORK: 2. 1 section assessment. Remember to check your answers using the appropriate Power Point in the Student Vault! WARM-UP: Give an example of an extensive property and an intensive property of a balloon. STAMPS: 2. 1 reading notes Done with the warm-up? Get started on the homework.

Chemistry Thursday 9/1/2016 HOMEWORK: 2. 2 Practice Problems (page 46 – LOOK NOW!) * Answers in back of book (WHAT PAGE? ), so self-correct (happy faces, cross-outs, etc. ) WARM-UP: Name two methods that can be used to separate substances that are mixed together. STAMPS: 2. 2 reading notes Done with the warm-up? Get started on the homework.

Chemistry Friday 9/2/2016 HOMEWORK: 2. 2 Section Assessment WARM-UP: What could you do to separate a mixture of chalk dust and small rocks? STAMPS: 2. 2 Practice Problems (#’s 9 & 10) Done with the warm-up? Get started on the homework.




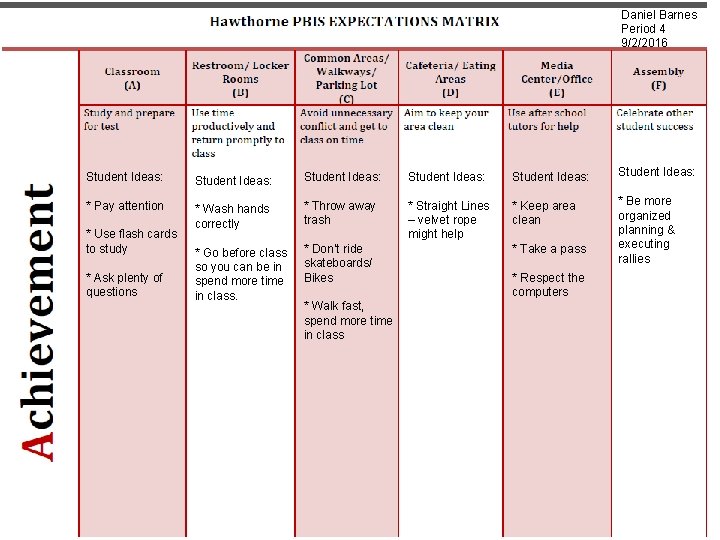
Daniel Barnes Period 4 9/2/2016 Student Ideas: Student Ideas: * Pay attention * Wash hands correctly * Throw away trash * Straight Lines – velvet rope might help * Keep area clean * Go before class so you can be in spend more time in class. * Don’t ride skateboards/ Bikes * Be more organized planning & executing rallies * Use flash cards to study * Ask plenty of questions * Walk fast, spend more time in class * Take a pass * Respect the computers

Chemistry Tuesday 9/6/2016 HOMEWORK: 2. 3 reading notes, practice problems (#’s 18 & 19 on page 51) [NOTE: When answering the warm-up below, make sure to impress me with your 2. 2 vocabulary knowledge. ] WARM-UP: You put sugar, motor oil, and water in a bottle. You shake it and let it sit for an hour. What is in the bottle? STAMPS: 2. 2 Section Assessment Done with the warm-up? Get started on the homework.

Chemistry Wednesday 9/7/2016 HOMEWORK: 2. 3 section assessment WARM-UP: What element is “Hg” the symbol for? How could it be the symbol for that element? STAMPS: 2. 3 reading notes, practice problems (#’s 18 & 19 on page 51) Done with the warm-up? Get started on the homework.

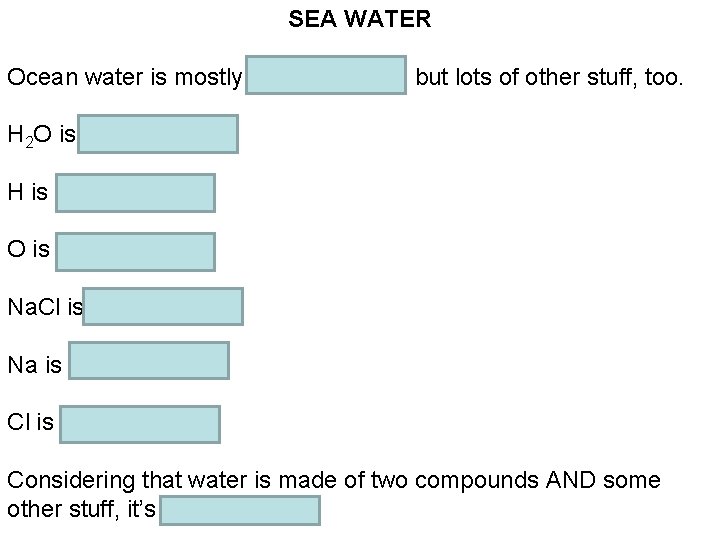
Chemistry Thursday 9/8/2016 HOMEWORK: 2. 4 reading notes WARM-UP: Is ocean water an element, a compound, or a mixture? Why? STAMPS: 2. 3 section assessment (#’s 20 -27) Done with the warm-up? Get started on the homework.

SEA WATER Ocean water is mostly H 2 O and Na. Cl, but lots of other stuff, too. H 2 O is a compound H is an element O is an element Na. Cl is a compound Na is an element Cl is an element Considering that water is made of two compounds AND some other stuff, it’s a mixture.

Chemistry Friday 9/9/2016 EXTRA CREDIT: Show me tonight’s homework noted in your planner before the tardy bell class participation bonus HOMEWORK: 2. 4 section assessment WARM-UP: A 100 -gram lump of charcoal burns, leaving behind a 13 -gram pile of ashes. Does this violate the law of conservation of matter? STAMPS: 2. 4 reading notes Done with the warm-up? Get started on the homework.

Chemistry Monday 9/12/2016 EXTRA CREDIT ONCE MORE: Show me tonight’s homework noted in your planner before the tardy bell class participation bonus HOMEWORK: Study ch 2 (and 1. 3!) for tomorrow’s ch 2 test WARM-UP: What happens during a chemical reaction? STAMPS: 2. 4 section assessment #’s 28 -34 Done with the warm-up? Get started on the homework.

Chemistry Tuesday 9/13/2016 HOMEWORK: Gather together your chapter 2 bookwork, lecture notes, video notes, and worksheets. The chapter 2 packet is due tomorrow. WARM-UP: Get out a blank piece of paper. Name, period and date in upper right hand corner. Title = “SCR 2” or “Chapter 2 Scratch” Get an answer document. Answer document title = “Chem 02” STAMPS: none today Stand by for the chapter 2 test.

“Form” = Version Your form is indicated by your red test packet #. Your red test packet # should be the same as your seat #. If your test # is this. . . then your form # is this: 1, 6, 11, 16, 21, 26, 31, 36 = Form 1 2, 7, 12, 17, 22, 27, 32, 37 = Form 2 3, 8, 13, 18, 23, 28, 33, 38 = Form 3 4, 9, 14, 19, 24, 29, 34, 39 = Form 4 5, 10, 15, 20, 25, 30, 35, 40 = Form 5

Chemistry Chapter 02 Screen Questions 9/13/2016 1. Compare and contrast the three main states of matter. Include in your discussion what atoms and molecules do in each state. 2. What evidence supported the scientific community in its abandonment of phlogiston theory? 3. Describe and explain a bottle containing a layer of gasoline sitting on top of a layer of salt water with sand on the bottom. 4. What is air made of? How could one separate air into its components? 5. Janelle reacts 10 g baking soda with 100 g vinegar in a cup and the combo has a mass of only 107 g after mixing. She concludes that 3 g of matter was destroyed. Please comment.

Chemistry Wednesday 9/14/2016 HOMEWORK: 13. 1 reading notes, Pr. Pr #’s 1 & 2 (TRY!) WARM-UP: Gather and organize your chapter 02 packet: * all chapter 2 bookwork, in order * elements/compounds/mixtures notes * mystery of matter video worksheet * anything I forgot? NO WARM-UPS IN PACKET! Also, no triple beam balance notes. We’re not done with them yet. . . STAMPS: none today

Chemistry: 13. 1: Read, take notes, prpr NO TALKING Headphones OK

Chemistry Thursday 9/15/2016 HOMEWORK: 13. 1 Pr. Pr (if not done yet), sxn assmt WARM-UP: How fast does an average oxygen molecule fly in km/h? How about in mi/h? (2 mi = 3 km, roughly) (This is the last warm-up of the 5 -wk WUp packet) STAMPS: 13. 1 reading notes, Pr. Pr #’s 1 & 2 (soft due date) Done with the warm-up? Get started on the homework.

Chemistry Chapter 02 Packet List due 9/14/2016 (soft) 2. 1 notes, section assessment – 2 pts 2. 2 notes, practice problems, section assessment – 3 pts 2. 3 notes, practice problems, section assessment – 3 pts 2. 4 notes, section assessment – 2 pts Elements/Compounds/Mixtures notes – 1 pt Mystery of Matter video notes – 1 pt Mystery of Matter worksheet – 2 pts (front & back) PBIS activity – 1 pt xcr (4 th period only) x/13 for grading purposes NPD = name, period, date (no points for sheet – add & resubmit)

Chemistry Friday 9/16/2016 HOMEWORK: ALL STUDENTS: 13. 1 undone math problems, if any. HONORS: Also read and take notes throughout the rest of chapter 13. Questions are optional. “ 10 Week Warm-Up Packet” WARM-UP: If the absolute air pressure in a car tire is 45 lb/in 2, what is it in mm Hg? STAMPS: 13. 1 section assessment (#’s 3 -7), prpr #’s 1 & 2 late okay Done with the warm-up? Get started on the homework.

Barnes' Grading System 50% Multiple choice tests 20% Scratch paper 10% Work 10% Labs 10% Class Participation

BORDERLINE GRADES * If you end the semester with a 59. 99%, you still get an “F”. I’m not going to knock you up to a “D”. * If you end the semester with a 60. 01%, you still get a “D”. I’m not going to knock you down to an “F”. If you’re dangerously close to the borderline, STEP ON THE GAS! MOVE! * Getting a good grade is a year-long process. Don’t slack off all semester and then expect a last-minute miracle.

Chemistry Monday 9/19/2016 HOMEWORK: ALL STUDENTS: 13. 2 reading notes HONORS: Also read and take notes throughout the rest of chapter 13. Questions are optional. (re-post of last hw) T = “ 10 Week Warm-Up Packet” (if not already titled) WARM-UP: How many k. Pa is 12 atm? STAMPS: ALL: 13. 1 math not yet stamped HONORS: Reading notes from 13. 2, 13. 3, 13. 4 (3 stamps!) Done with the warm-up? Get started on the homework.

Chemistry Tuesday 9/20/2016 HOMEWORK: 4. 1 read, take notes WARM-UP: At the top of a mountain, the mercury column in an old barometer is only 450 mm tall. What is the pressure there in lb/in 2? STAMPS: any unstamped ch 13 except 13. 1 notes Done with the warm-up? Get started on the homework.

HEY! Period 3 Kaiser Permanente assembly people: Click through my “States of Matter” power point on hhscougars. org (It’s on my “Chemistry Power Points” page. )

UNIT CONVERSION STEPS (including pressure conversions, of course) 1. 2. 3. 4. 5. 6. 7. Put original amount over one Times a fraction Old unit on the bottom New unit on the top Put in numbers so bottom equals top Cancel old units Do the math

Chemistry Wednesday 9/21/2016 HOMEWORK: 4. 1 section assessment WARM-UP: What does the word “atom” mean in Greek? STAMPS: 4. 1 reading notes + last possible chance for chapter 13 math mercy stamps (Ch 13 packet due today) Done with the warm-up? Get started on the homework.

Chapter 13 Packet List due 9/21/16 * All chapter 13 bookwork, stamped or unstamped * States of Matter notes * Syringe & Water notes * Anything I forgot? Do NOT include the ch 02 Quiz Show notes. We’ll finish those later and turn them in later. Yeah, not much to it, huh? Remember, ch 13 will be a full-strength part of ch 4 test. . . NOTE: Honors was required to take notes in all four sections. Normal was required to take notes in only the first two.

Chemistry Thursday 9/22/2016 HOMEWORK: 4. 2 reading notes; finish Static Electricity Lab (captioned/labeled pictures on back of worksheet) WARM-UP: Why do you suppose solids are so solid? STAMPS: 4. 1 section assessment (#’s 1 -7) Done with the warm-up? Get started on the homework.

Chapter 13 Detailed Packet List due 9/21/16 * All chapter 13 bookwork, stamped or unstamped * 13. 1 reading notes N H * 13. 1 practice problems N H * 13. 1 section assessment N H * 13. 2 reading notes N H * 13. 2 section assessment * 13. 3 reading notes H * 13. 3 section assessment * 13. 4 reading notes H * 13. 4 section assessment * States of Matter notes N H * Syringe & Water notes N H -----------------------------------Normal = x/6 Honors = x/8 Points possible, including xcr = 11

Chemistry Friday 9/23/2016 HOMEWORK: 4. 2 section assessment WARM-UP: What was the first subatomic particle ever discovered? Any idea why we discovered that one first? STAMPS: 4. 2 reading notes Done with the warm-up? Get started on the homework.

Because of Club Grub, Food is OK today

Chemistry Monday 9/26/2016 HOMEWORK: finish the Static Electricity Lab if not already done. Watch the debate. WARM-UP: Compare and contrast gravity and the electrostatic force. STAMPS: 4. 2 section assessment (#’s 8 -14) Done with the warm-up? Get started on the homework.

Compare and Contrast Gravity and the Electrostatic Force * Both are field forces. Two objects do not need to touch each other to feel each other’s forces. * Both forces get weaker with distance. * The strength of the gravitational force depends on the masses of the two objects, whereas the strength of the electrostatic force depends upon the electric charges of the two objects. * Gravity is always attractive, whereas the electrostatic force can be either attractive (+ -) or repulsive (+ + or - - ). * Gravity is significantly powerful with larger objects, including people and planets, whereas it is insignificant, compared to the electrostatic force, with microscopic objects.

Chemistry Tuesday 9/27/2016 HOMEWORK: No new homework tonight. Expect some soon. WARM-UP: What are the masses, charges, and locations of the three main subatomic particles? STAMPS: nothing stamped today Done with the warm-up? Get started on the homework. Wait. What homework?

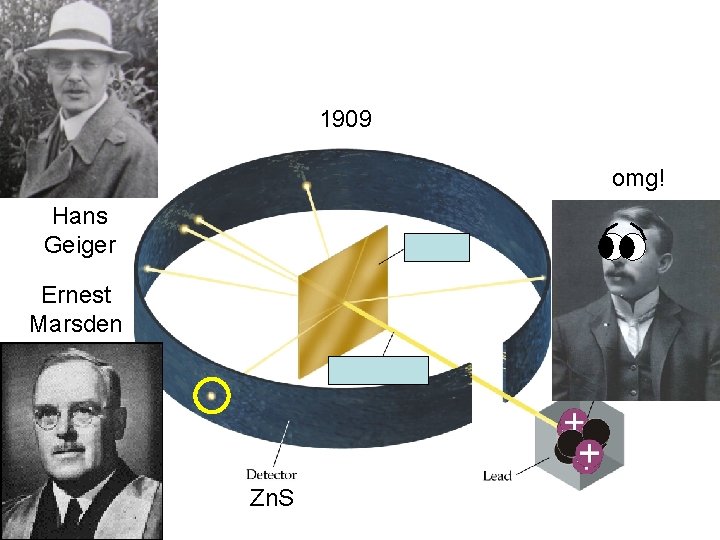
Chemistry Wednesday 9/28/2016 HOMEWORK: 4. 3 reading notes, practice problems (answers in back of book) WARM-UP: What happened in the gold foil experiment? What did Rutherford conclude as a result? STAMPS: nothing stamped today Done with the warm-up? Get started on the homework.

1909 omg! Hans Geiger Ernest Marsden Zn. S

Chemistry Thor’s Day 9/29/2016 HOMEWORK: 4. 3 section assessment, Atom Rules Determination Activity WARM-UP: Why are the atomic masses on the periodic table almost never whole numbers? STAMPS: 4. 3 reading notes & practice problems #15 -24 Done with the warm-up? Get started on the homework.

Why aren’t the atomic masses whole numbers? Reason #1: Proton mass = 1. 007 amu Neutron mass = 1. 009 amu Electron mass = 0. 000549 amu None of these #’s are whole numbers. Reason #2: Atomic masses on the periodic tables are the weighted average of all the atomic masses of the naturallyoccurring isotopes of any given element. Reason #3: The mass defect. When atomic nuclei are assembled out of protons & neutrons, energy gets turned into matter & vice versa (according to e = mc 2).

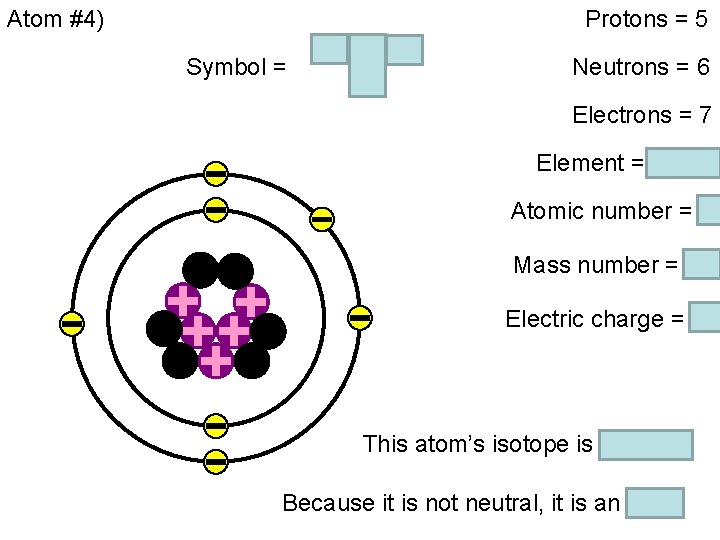
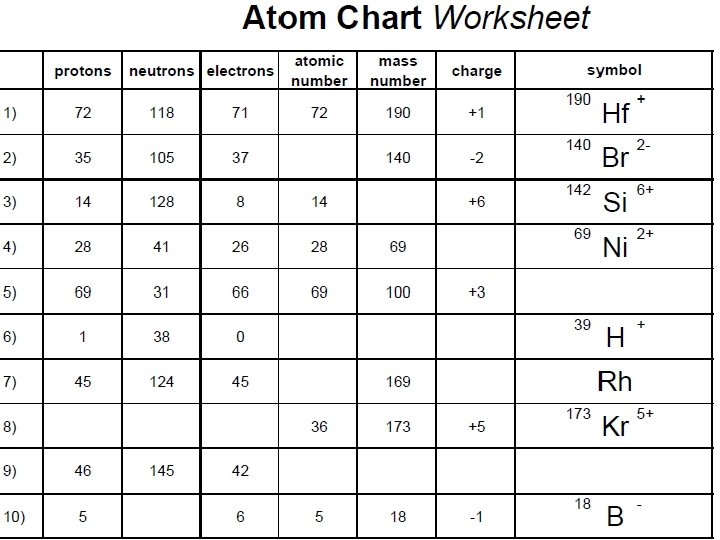
Chemistry Friday 9/30/2016 HOMEWORK: Atom Chart Worksheet. Please pick one up now from the box on the front table! (NOTE: Don’t peek at the answers until you’ve tried to fill in the boxes on the front all on your own. ) WARM-UP: What can you tell me about an atom that has five protons, six neutrons, and seven electrons? STAMPS: 4. 3 section assessment #’s 25 -33 Done with the warm-up? Get started on the homework.

Atom #4) Symbol = 11 B 2 - Protons = 5 Neutrons = 6 Electrons = 7 Element = boron Atomic number = 5 Mass number = 11 Electric charge = -2 This atom’s isotope is boron-11 Because it is not neutral, it is an “ion”.

5. Which of the following animals has fins? A. cat B. dog C. bear D. fish


RETURN ANSWER DOCUMENTS HERE Thank you!

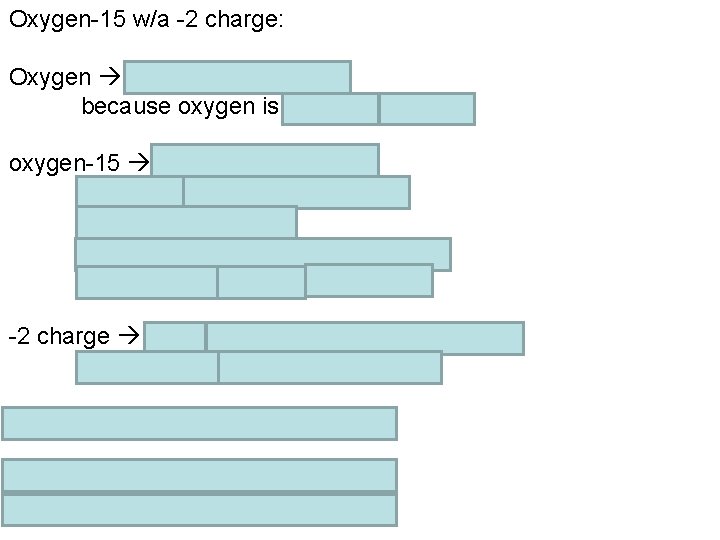
Chemistry Monday 10/3/2016 HOMEWORK: Study for Wednesday’s ch 13 / ch 04 test. Meet in the Media Center computer lab tomorrow. WARM-UP: What should a oxygen-15 atom with a 2 - charge be made of? STAMPS: none today – worksheet not being stamped Done with the warm-up? Get started on the homework.

Oxygen-15 w/a -2 charge: Oxygen must have 8 protons because oxygen is atomic # 8 oxygen-15 has a mass # of 15 mass # = protons + neutrons # of protons = 8, so # neutrons = mass # - # of protons # neutrons = 15 – 8 = 7 neutrons -2 charge more electrons (-) than protons (+) 8 protons, so 8 + 2 = 10 electrons 8 protons, 7 neutrons, 10 electrons. charge = protons – electrons = protons - charge

Oxygen-15 w/a -2 charge: Oxygen must have 8 protons because oxygen is atomic # 8 oxygen-15 has a mass # of 15 mass # = protons + neutrons # of protons = 8, so # neutrons = mass # - # of protons # neutrons = 15 – 8 = 7 neutrons -2 charge more electrons (-) than protons (+) 8 protons, so 8 + 2 = 10 electrons 8 protons, 7 neutrons, 10 electrons. charge = protons – electrons = protons - charge

Chemistry Tuesday 10/4/2016 HOMEWORK: Study for tomorrow’s ch 04 / 13 test WARM-UP: Open Internet Explorer. Browse to hhscougars. org. Locate my home page. Go to my chemistry power points page. Open the file called “Atom Identification DRB. ppt”. Hit F 5 or shift-F 5 to go into slideshow mode Entitle some paper “Atom ID Notes”. Take notes as you click. Before each click, try to guess what you’re going to see next. STAMPS: nothing new today except old math Done with the warm-up? Open and click through “Periodic Table Introduction DRB. ppt”

Chemistry Wednesday 10/5/2016 HOMEWORK: Gather your ch 04 bookwork, lecture notes, worksheets. Ch 04 packet due tomorrow. WARM-UP: Answer doc title = Chemistry 04 Scratch paper title = SCR 04 STAMPS: nothing new today bit last chance for stamps for old ch 04 math Done with the warm-up? Stand by to begin the test.

“Form” = Version Your form is indicated by your red test packet #. Your red test packet # should be the same as your seat #. If your test # is this. . . then your form # is this: 1, 6, 11, 16, 21, 26, 31, 36 = Form 1 2, 7, 12, 17, 22, 27, 32, 37 = Form 2 3, 8, 13, 18, 23, 28, 33, 38 = Form 3 4, 9, 14, 19, 24, 29, 34, 39 = Form 4 5, 10, 15, 20, 25, 30, 35, 40 = Form 5

Chapter 04 and 13 Screen Questions 10/5/2016 1. What happened when you pulled back the grey plunger in the syringe full of water? Why? 2. List the steps for doing a unit conversion. Use these steps to convert 45 lbs/in 2 to mm Hg. (You may round a bit. ) 3. What does the word “atom” mean in Greek? In retrospect, was this the best name to pick? Why/why not? 4. What happened during Rutherford’s famous “gold foil” experiment? What did he conclude from these results? 5. Compare and contrast gravity and the electrostatic force. 6. Why aren’t atomic masses on the periodic table usually whole numbers?

Chemistry Thursday 10/6/2016 HOMEWORK: 25. 1 reading notes, section assessment – will be stamped Monday 10/10/16 WARM-UP: Gather together your ch 04 bookwork, lecture notes, and worksheets. A packet list will appear shortly to show order. STAMPS: last chance for 4. 3 prpr & 4. 3 sxn assmt Done with the warm-up? Get started on the hw

Chapter 04 Packet List due 10/06/2016 * 4. 1 reading notes (MIGHT BE IN CH 13 PACKET! GET IT!) * 4. 1 section assessment * 4. 2 reading notes * 4. 2 section assessment * 4. 3 reading notes * 4. 3 practice problems * 4. 3 section assessment * Atomic Structure presentation notes * Atom Identification presentation notes * Atom Chart worksheet (empty boxes on front/answers in back) * Ch 04 Quiz Show notes (if you took any in the computer lab) * Per 4 only: PBIS scenario re-write (on back of matrix)

Chemistry Monday 10/10/2016 HOMEWORK: 25. 2 reading notes, practice problems WARM-UP: Compare & contrast alpha, beta, & gamma radiation. STAMPS: 25. 1 reading notes, section assessment Done with the warm-up? Get started on the hw

Chemistry Tuesday 10/11/2016 HOMEWORK: 25. 2 section assessment WARM-UP: What is special about uranium? STAMPS: 25. 2 reading notes, practice problems #’s 7 & 8 Done with the warm-up? Get started on the hw

URANIUM It’s named after Uranus. It’s the largest, naturally-occurring element. All elements with an atomic number larger than uranium must be forced into existence by man, artificially. All of its isotopes are radioactive, being alpha emitters, but that alpha decay is just the first step in a long series of decays, some of which give off beta and/or gamma particles, so don’t assume you can protect yourself from uranium with just a piece of paper. The isotope uranium-235 is “fissile” and can be used to power an atom bomb, trigger fusion in a hydrogen bomb, or power a nuclear power plant.
![Property Neutron Proton Electron Charge 0 1 1 Nucleus center of atom electron cloud [Property] Neutron Proton Electron Charge 0 +1 -1 Nucleus (center of atom) electron cloud,](https://slidetodoc.com/presentation_image/53ab5db144cb996b09fa006ea409256d/image-77.jpg)
[Property] Neutron Proton Electron Charge 0 +1 -1 Nucleus (center of atom) electron cloud, orbiting the nucleus (outer part of atom) About 1 amu 0. 0005 amu = 1/1840 amu = MUCH lighter than the other two Location Mass Nucleus (center of atom) About 1 amu

Chemistry Wednesday 10/12/2016 HOMEWORK: none. We need to digest 25. 2 a little more before moving on. WARM-UP: Is there anything GOOD that radioactive atoms do? STAMPS: 25. 2 section assessment (#’s 9 -14) Done with the warm-up? Work ahead into 25. 3 anyway.

Chemistry Thursday 10/13/2016 HOMEWORK: none. not done w/25. 2 yet. WARM-UP: What happens to a radioactive atom when one half-life of time goes by? STAMPS: old math (unstamped 25. 2 prpr, sxn assmt) Done with the warm-up? 1. Get out the yellow 25. 1 reading guide 2. Finish the yellow 25. 1 reading guide if not done yet 3. Work ahead into 25. 3 & 25. 4 anyway. Do not just chat. That wastes valuable class time.

Chemistry Friday 10/14/2016 HOMEWORK: none – but don’t get used to it. WARM-UP: What does alpha decay do to a nucleus? What does beta decay do to a nucleus? NOTE: This is the last warm-up of the 10 -week warm-up packet! STAMPS: old math (unstamped 25. 2 prpr, sxn assmt) Done with the warm-up? 1. Get out the yellow 25. 1 reading guide 2. Finish the yellow 25. 1 reading guide if not done yet 3. Work ahead into 25. 3 & 25. 4 anyway.

Chemistry Monday 10/17/2016 HOMEWORK: Radioactive Decay Series Worksheet WARM-UP: Put name, period and date on a brand new sheet of paper. Title it “ 15 -Week Warm-Up Packet” Skip a line Write today’s date again. Copy the following question and answer with CS/CSF: Q: How can an atom bomb hurt people? STAMPS: old math (unstamped 25. 2 prpr, sxn assmt) Done with the warm-up? Get started on the hw.

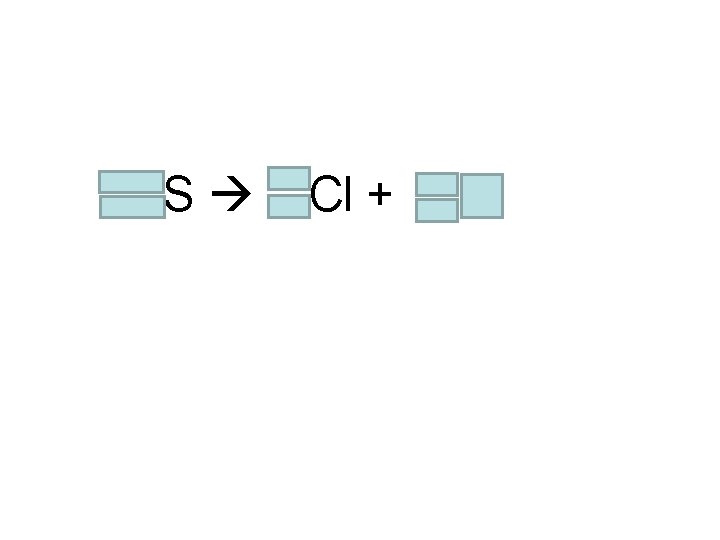
Chemistry Tuesday 10/18/2016 HOMEWORK: Finish the Radioactive Decay Simulation Lab WARM-UP: Skip a line after yesterday’s warm-up. Write today’s date. On the next line, copy the following question: “A sulfur-35 atom mysteriously turns into a chlorine-35 atom. How could that happen? ” Answer the question with one or more complete sentences. STAMPS: old math (unstamped 25. 2 prpr, sxn assmt) Done with the warm-up? Read ahead into 25. 3, 25. 4

35 16 S 35 17 Cl + 0 -1 e

Chemistry Wednesday 10/19/2016 HOMEWORK: 25. 3 reading notes, section assessment WARM-UP: none today (due to PSAT) STAMPS: none today

Chemistry Thursday 10/20/2016 HOMEWORK: All classes: 25. 4 reading notes Honors only: 25. 4 section assessment WARM-UP: How does one calculate an average? What is the average of 23, 47, 19, 5, 0, and 7? STAMPS: 25. 3 reading notes, section assessment (#’s 15 -20) Done with the warm-up? Work on the lab/start the hw.
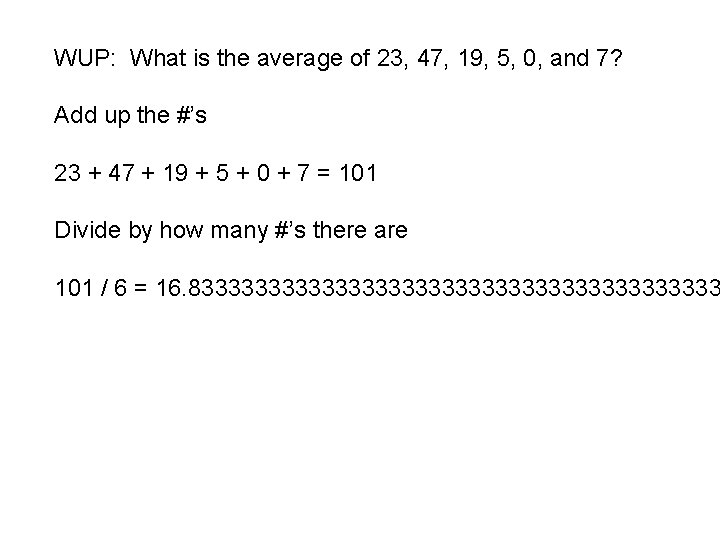
WUP: What is the average of 23, 47, 19, 5, 0, and 7? Add up the #’s 23 + 47 + 19 + 5 + 0 + 7 = 101 Divide by how many #’s there are 101 / 6 = 16. 833333333333333333333

Graphing Expectations in Mr. Barnes’ Class 1. Graph takes up whole page 2. One inch margins 3. Title of graph = “[y axis variable] vs [x axis variable]” 4. Each axis titled & numbered 5. Lower left hand corner is 0, 0. . . usually 6. Number w/consistent rhythm 7. Number the lines, not the squares

Chemistry Friday 10/21/2016 HOMEWORK: gather together bookwork, worksheets, and lecture notes for chapter 25. Packet due Monday. WARM-UP: Get a csa answer document. NPD in upper right hand corner. Write and bubble your ID digits. Stand by for the CSA. STAMPS: 25. 4 reading notes, section assessment (#’s 21 -24)

Chemistry Monday 10/24/2016 HOMEWORK: 5. 1 reading notes, section assessment WARM-UP: Get out a pencil. Get out your Nuclear Chemistry Notes Draw a thick horizontal line right after the end of your notes. Skip a line. Write “CSA 1 Reflection” and put a box around it. Get ready to take some good notes in your reflection. . . STAMPS: 25. 4 reading notes, section assessment (#’s 21 -24) (Especially period 1!) Done with the warm-up? Work on the homework

CSA Reflection Get out a pencil. Get out your Nuclear Chemistry Notes. Draw a thick horizontal line right after the end of your notes. Skip a line. Write “CSA 1 Reflection” and put a box around it. Get ready to take some good notes in your reflection as we go over the answers and you bubble your points. As we go over the CSA, keep the following in mind: * The purpose of this exercise is for teachers to find out what we learned well and what we didn’t, so that we can teach and learn better. * Your grade on this assignment has nothing to do with how many questions you got right. Just sincere effort. * Be honest about how many points you deserve, but. . . * Don’t agonize too much. Follow the rubric, but go with your gut. * Bubble in pencil in case you change your mind.

Chapter 25 Packet List due 10/24/2016 – okay, well, really 10/25/16 for most periods due to delays * 25. 1 reading notes * 25. 1 section assessment * 25. 2 reading notes * 25. 2 practice problems (last day for stamps = today!) * 25. 2 section assessment (last day for stamps = today!) * 25. 3 reading notes * 25. 3 section assessment * 25. 4 reading notes (stampable today! ) * 25. 4 section assessment (stampable today! ) * 25. 1 reading guide (yellow) * Radioactive Decay Series Worksheet * Nuclear Chemistry Notes * CSA-1 Reflection * Anything I forgot?

Chemistry Tuesday 10/25/2016 HOMEWORK: 5. 2 reading notes, practice problems (HARD) WARM-UP: Who was the first scientist to propose that electrons orbit the nucleus of an atom? STAMPS: 5. 1 reading notes, section assessment Done with the warm-up? Finish scoring your CSA – in PENCIL. Done with that? Work on the homework.

Hantaro Nagaoka August 19, 1865 – December 11, 1950 Came up with a solar system model of the atom before Rutherford did! (more of a rings-ofsaturn model, but still based on eoribiting a massive, positive center)

Chemistry Wednesday 10/26/2016 HOMEWORK: None tonight. We need to work on electron configurations in class before we go any further at home in the book. WARM-UP: Compare and contrast the Rutherford and Bohr models of the atom. ALSO! Look at the windows & the helium tube through the prism. Rotate until you see colored stripes. Pass it on. STAMPS: 5. 2 reading notes, practice problems Done with the warm-up? Read ahead in 5. 3 & take notes. You’ll have to read it soon, anyway. Don’t sweat the math.

Chemistry Thursday 10/27/2016 HOMEWORK: None yet. One more night off. PRE-WARM-UP: Feel free to grab a prism and look at the glowing hydrogen tube up close. WARM-UP: Why does the hydrogen tube glow a different color than the helium did? STAMPS: 5. 2 practice problems (late but ok) Done with the warm-up? Read ahead in 5. 3 & take notes. You’ll have to read it soon, anyway. Don’t sweat the math, unless you’re honors.

Chemistry Friday 10/28/2016 HOMEWORK: ALL STUDENTS: 5. 2 section assessssment HONORS: 5. 3 all as well. There is math. PRE-WARM-UP: Today’s tube: neon. Grab a prism and rotate it if you have a mind to. WARM-UP: What is the Heisenberg uncertainty principle? STAMPS: 5. 2 practice problems (late but ok) Done with the warm-up? Work ahead in the book.

Chemistry Monday 10/31/2016 HOMEWORK: Chapter 5 assessment questions #’s 30 -39 WARM-UP: How many electrons can fit in an orbital? Under what circumstances? STAMPS: ALL STUDENTS: 5. 2 practice problems (late but ok) 5. 2 section assessment (#’s 10 -13) HONORS: 5. 3 all as well. (notes, prpr 14 & 15, sa 16 -21) Done with the warm-up? Get started on the homework.

Chemistry Monday 10/31/2016 HOMEWORK: Chapter 5 assessment questions #’s 30 -39 WARM-UP: How many electrons can fit in an orbital? Under what circumstances? STAMPS: ALL STUDENTS: 5. 2 practice problems (late but ok) 5. 2 section assessment (#’s 10 -13) HONORS: 5. 3 all as well. (notes, prpr 14 & 15, sa 16 -21) Done with the warm-up? Get started on the homework. =

Chemistry Tuesday 11/1/2016 HOMEWORK: Study for tomorrow’s ch 05/25 test. 1/3 ch 05 1/3 ch 25 1/3 old stuff (sxn 01. 3; ch’s 02, 13, 04) WARM-UP: How many electrons can fit into an “s” sublevel? A “p” sublevel? “d”? “f”? STAMPS: ch 05 assessment #’s 30 -39 Done with the warm-up? Review ch 05 & 25. Test tomorrow.

Chemistry Wednesday 11/2/2016 HOMEWORK: Gather your chapter 05 bookwork and lecture notes. Packet due tomorrow. WARM-UP: Get an answer document. Name/period/date. ID#. Answer doc title = “Chem 05” Scratch paper title = “SCR 05” Get started on the test. No need to wait for the bell. STAMPS: Anything from ch 05 involving numbers

“Form” = Version Your form is indicated by your red test packet #. Your red test packet # should be the same as your seat #. If your test # is this. . . then your form # is this: 1, 6, 11, 16, 21, 26, 31, 36 = Form 1 2, 7, 12, 17, 22, 27, 32, 37 = Form 2 3, 8, 13, 18, 23, 28, 33, 38 = Form 3 4, 9, 14, 19, 24, 29, 34, 39 = Form 4 5, 10, 15, 20, 25, 30, 35, 40 = Form 5

Chapter 05 & 25 Screen Questions 11/2/2016 1. Write the electron configurations and orbital diagrams for the following: a. nitrogen b. krypton c. uranium 2. Why do neon, helium, and hydrogen glow different colors? 3. A box contains 200 -grams of pure protactinium-234. Twenty hours later, the box contains 25 grams of protactinium-234 and 175 grams of uranium-234. What happened? 4. Compare and contrast radioactive decay, nuclear fission, and nuclear fusion. 5. Are you for or against nuclear power? Why? 6. Tell the story of how atomic theory evolved over time.

Chemistry Thursday 11/3/2016 HOMEWORK: 6. 1 read & take notes WARM-UP: What is weird about the electron configuration 1 s 22 p 43 s 1? STAMPS: Anything from ch 05 involving numbers LAST CHANCE FOR CHAPTER 05 STAMPS! Done with the warm-up? Start the homework.

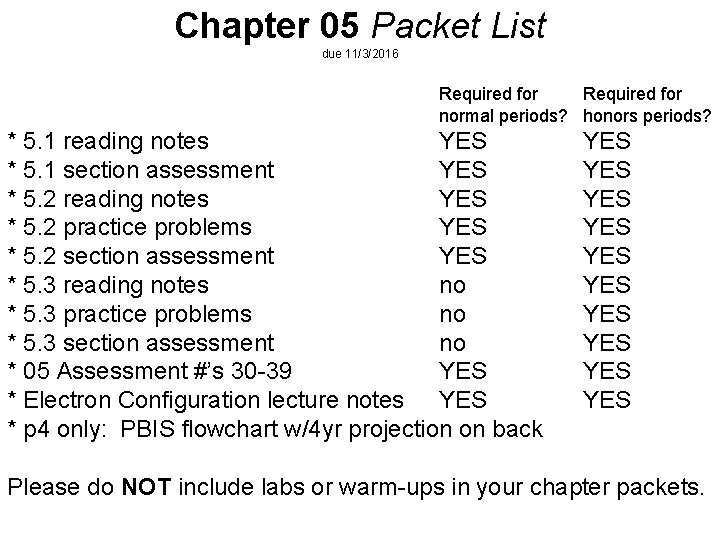
Chapter 05 Packet List due 11/3/2016 Required for normal periods? honors periods? * 5. 1 reading notes YES * 5. 1 section assessment YES * 5. 2 reading notes YES * 5. 2 practice problems YES * 5. 2 section assessment YES * 5. 3 reading notes no * 5. 3 practice problems no * 5. 3 section assessment no * 05 Assessment #’s 30 -39 YES * Electron Configuration lecture notes YES * p 4 only: PBIS flowchart w/4 yr projection on back YES YES YES Please do NOT include labs or warm-ups in your chapter packets.

05 Reflection Instructions NPD in URHC on new sheet of paper Title = “ 05 Reflection” Go to “version table” based on which “form” you used. Highest-scoring person at table is “version leader”. Version leader recites answer key to group. As answers are recited, students somehow record which question #’s they got wrong and what the correct answers were. REFLECTION: For each question you got wrong, write on your reflection paper why your wrong answer was wrong and why the right answer was right. Vow to yourself to never get that question wrong again.

Chemistry Friday 11/4/2016 HOMEWORK: 6. 1 section assessment WARM-UP: Who made the first periodic table and how did he arrange the elements on it? STAMPS: 6. 1 reading notes Done with the warm-up? Start the homework.

Chemistry Monday 11/7/2016 HOMEWORK: 6. 2 reading notes, practice problems WARM-UP: What do all metals have in common? STAMPS: 6. 1 section assessment #’s 1 -7 Done with the warm-up? Start the homework.

Chemistry Tuesday 11/8/2016 HOMEWORK: 6. 2 section assessment WARM-UP: What do all alkali metals have in common? STAMPS: 6. 2 notes, practice problems #’s 8 & 9 Done with the warm-up? Start the homework. No. Really. Start the homework. Actually. For realsies.

Chemistry Wednesday 11/9/2016 HOMEWORK: nothing tonight WARM-UP: What is true of the electron configurations of all noble gases? STAMPS: 6. 2 section assessment (#’s 10 -15) Done with the warm-up? Think about what you just did, America.

Chemistry Thursday 11/10/2016 HOMEWORK: 6. 3 reading notes WARM-UP: Based on the videos, what are the two most extreme elements; where are they on the PT? STAMPS: none today Done with the warm-up? Do the homework. 15 -Week Warm-Up Packet due today All warm-ups from 10/17 -11/10/16

Chemistry Monday 11/14/2016 HOMEWORK: 6. 3 section assessment Get out a brand new, blank piece of paper. Name, period, & date in upper right hand corner. Title = “ 20 Week Warm-Up Packet” Skip a line & write today’s date again. Copy the following question and answer it with one or more complete sentences: WARM-UP: What happens to a neutral atom when it loses an electron? STAMPS: 6. 3 reading notes

Chemistry Tuesday 11/15/201 6 HOMEWORK: Use webelements. com to finish the atomic radius graph. (Use the “calculated” radius. ) WARM-UP: Get out the warm-up sheet you started yesterday. Skip a line after the last warm-up. Write today’s date. Copy and answer the following question: What is the radius of an atom? ( _DQA_DQA_DQA_DQA_DQA. . . ) STAMPS: 6. 3 section assessment = #’s 16 -23 Done with the warm-up? Do the homework.