SOLUTION CHEMISTRY C Adhesion Adhesion is an attraction















- Slides: 23

SOLUTION CHEMISTRY


C. Adhesion • Adhesion is an attraction between different substances. • Water molecules are attracted to many other polar substances. • Adhesion causes a behavior known as capillary action. Cohesion causes water to form drops Surface tension causes them to be round Adhesion keeps the drops in place

D. Density in Liquid & Solid States As the temperature decreases, the forming of hydrogen bonds creates a crystalline structure which is very "open”. This “openness” created actually lowers the density of water as it freezes. • The empty space between the molecules lowers the over all mass for a given volume. • Which state of water has the greatest density? LIQUID E. The Universal Solvent • The polarity of water enables many substances to dissolve into it. • Ionic compounds and polar molecules dissolve best in water. (Ex: salt) • Nonpolar molecules do not dissolve well in water. (Ex: Oil) • The polar nature of water, which is very strong, causes water to be considered the greatest dissolving agent known to man.

*** PRACTICE PROBLEMS: 1 Which property of water causes attraction between molecules of liquid water? 2 Which state of water has the greatest density and why? 3 Describe the property of water that allows fish to survive through severe winters:

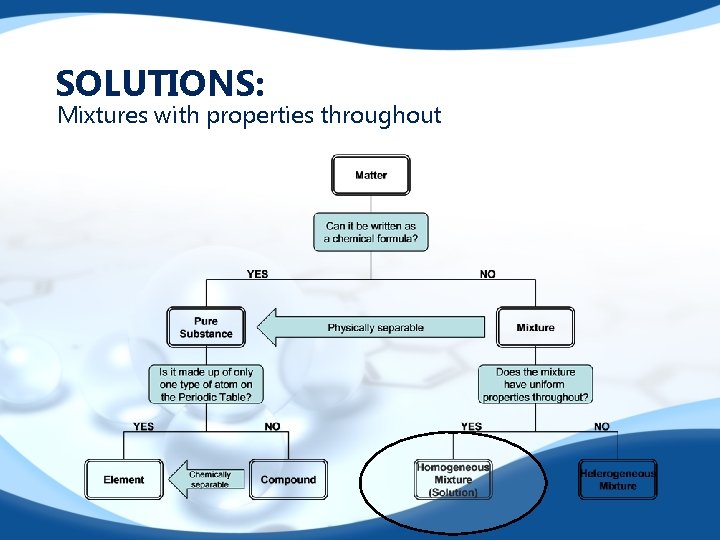
SOLUTIONS: Mixtures with properties throughout

Solutions. . . Need to know… 1. Substances that are capable of being dissolved are called soluble. 2. Substances that are incapable of being dissolved are called insoluble. 3. The dissolving medium in a solution is called thesolvent. (what is doing the dissolving)……. usually water, but not always!!!! 4. The substance dissolved in a solution is called thesolute. (what is being dissolved) 5. Solutions may exist as solids, liquids or gases. **All solution are not necessarily liquids!!! 6. Miscible liquids are able to dissolve freely in one another in any proportion. 7. Immiscible liquids are not soluble in each other(forms layers). Miscible and immiscible refer toliquid-liquid solutions, only.

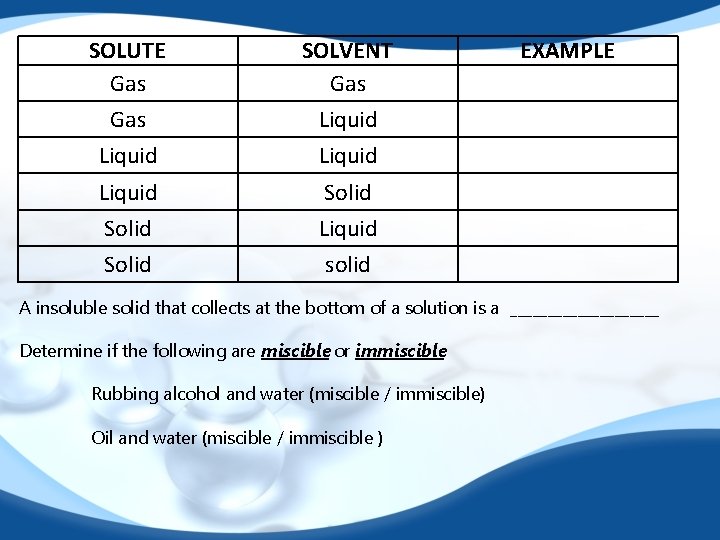
SOLUTE Gas SOLVENT Gas Liquid Solid Liquid Solid solid EXAMPLE A insoluble solid that collects at the bottom of a solution is a __________ Determine if the following are miscible or immiscible Rubbing alcohol and water (miscible / immiscible) Oil and water (miscible / immiscible )

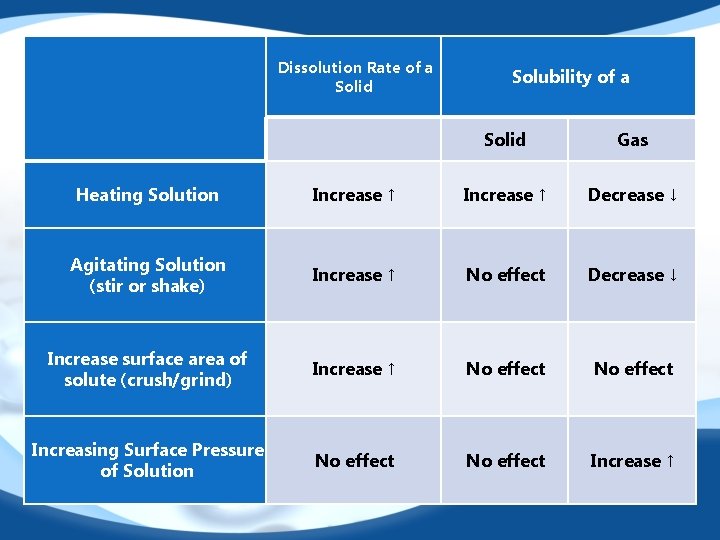
“Like Dissolves Like” 1. Polar and ionic solutes dissolve inpolar solvents. 2. Non-polar solutes dissolve in non-polar solvents. 3. Most compounds are either polar or ionic. Water is a polar solvent. Therefore water is universal solvent Rate of Dissolution: refers to how quickly a solute dissolves Solubility: refers to the amount of solute that can be dissolved at a specific temperature.

Dissolution Rate of a Solid Solubility of a Solid Gas Heating Solution Increase ↑ Decrease ↓ Agitating Solution (stir or shake) Increase ↑ No effect Decrease ↓ Increase surface area of solute (crush/grind) Increase ↑ No effect Increasing Surface Pressure of Solution No effect Increase ↑

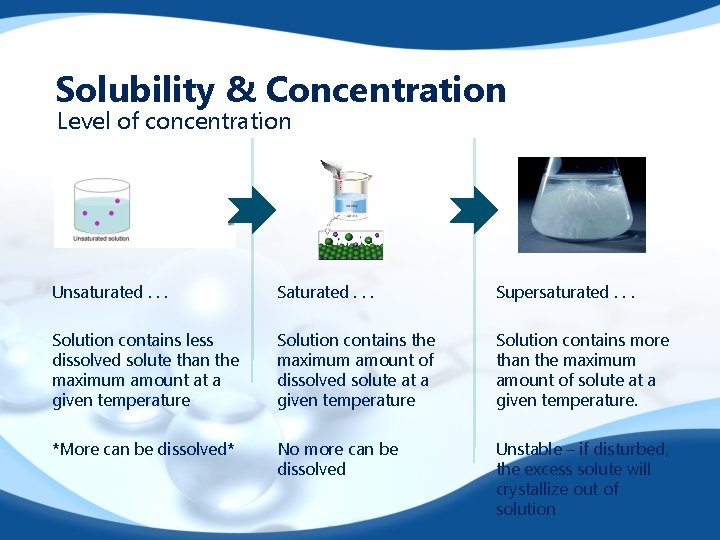
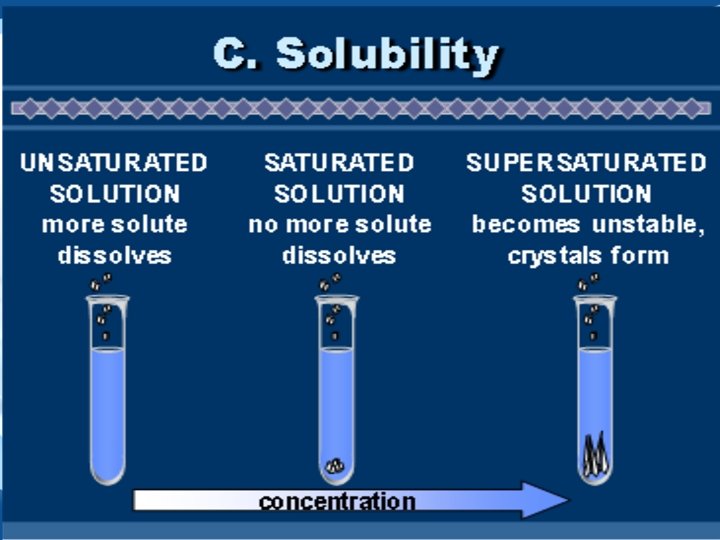
Solubility & Concentration Level of concentration gdsdf Unsaturated. . . Supersaturated. . . Solution contains less dissolved solute than the maximum amount at a given temperature Solution contains the maximum amount of dissolved solute at a given temperature Solution contains more than the maximum amount of solute at a given temperature. *More can be dissolved* No more can be dissolved Unstable – if disturbed, the excess solute will crystallize out of solution


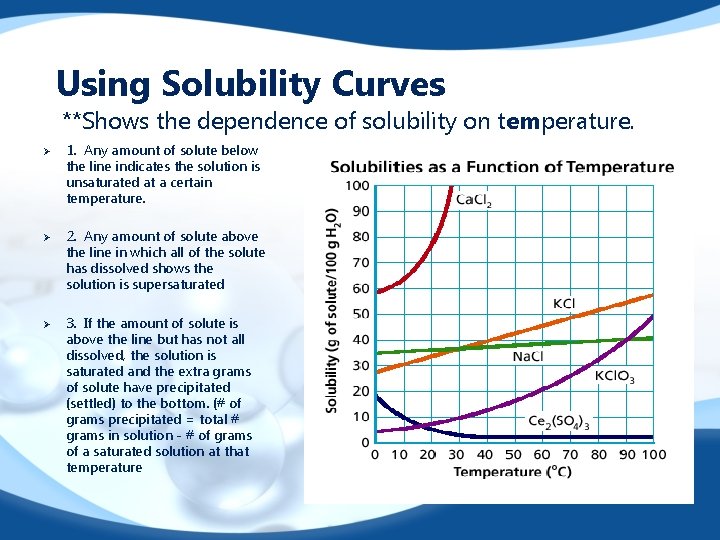
Using Solubility Curves **Shows the dependence of solubility on temperature. Ø Ø Ø 1. Any amount of solute below the line indicates the solution is unsaturated at a certain temperature. 2. Any amount of solute above the line in which all of the solute has dissolved shows the solution is supersaturated 3. If the amount of solute is above the line but has not all dissolved, the solution is saturated and the extra grams of solute have precipitated (settled) to the bottom. (# of grams precipitated = total # grams in solution - # of grams of a saturated solution at that temperature

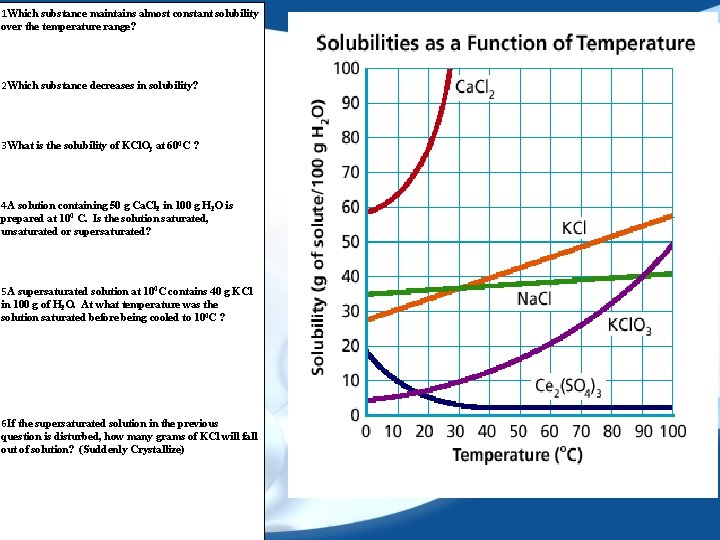
1 Which substance maintains almost constant solubility over the temperature range? 2 Which substance decreases in solubility? 3 What is the solubility of KCl. O 3 at 600 C ? 4 A solution containing 50 g Ca. Cl 2 in 100 g H 2 O is prepared at 100 C. Is the solution saturated, unsaturated or supersaturated? 5 A supersaturated solution at 100 C contains 40 g KCl in 100 g of H 2 O. At what temperature was the solution saturated before being cooled to 100 C ? 6 If the supersaturated solution in the previous question is disturbed, how many grams of KCl will fall out of solution? (Suddenly Crystallize)

Electrolytes: A substance that dissolves in water to make a solution that can conduct electrical current q q A. Any soluble, ionic compound is an electrolyte B. Some highly polar covalent molecules, such as strong acids, are also electrolytes. Questions to ask to determine if a substance is an electrolyte: q q Ca. Cl 2 H 2 SO 4 Na. OH Is the substance ionic? Is the substance soluble in water? (dissolve)

***DISSOCIATION REACTIONS (Only occur if a substance is soluble (able to be dissolved) in water. Determine if the following compounds are electrolytes. If they are, write the dissociation reaction: 1 Ca. Cl 2 2 H 2 SO 4 3 Na. OH 4 Li 3 PO 4 5 Ba. SO 4

CONCENTRATION – measure of the amount of solute in a given amount of solvent or solution. MOLARITY – (M) is the number of moles of solute in one liter of solution. (any solvent, not just water). If you are given mass, you will need to divide by the molar mass in order to find mol of solute. Molarity is designated by using the capital “M” directly after the number. refers to molarity. MOLAR **As you increase molarity, the solution is more CONCENTRATED……low molarity means you have a dilute solution, and high molarity means you have a CONCENTRATED solution

EXAMPLES: 1. What is the molarity of a solution that contains 1. 54 mol Na. Cl in 3. 5 L solution? 2. How many moles of HCl are required to prepare 0. 8 L of a 0. 5 M HCl solution? 3. If 35. 8 g of Li. OH are dissolved in enough water to make 750 ml of solution, what is the molar concentration (molarity) of the solution?

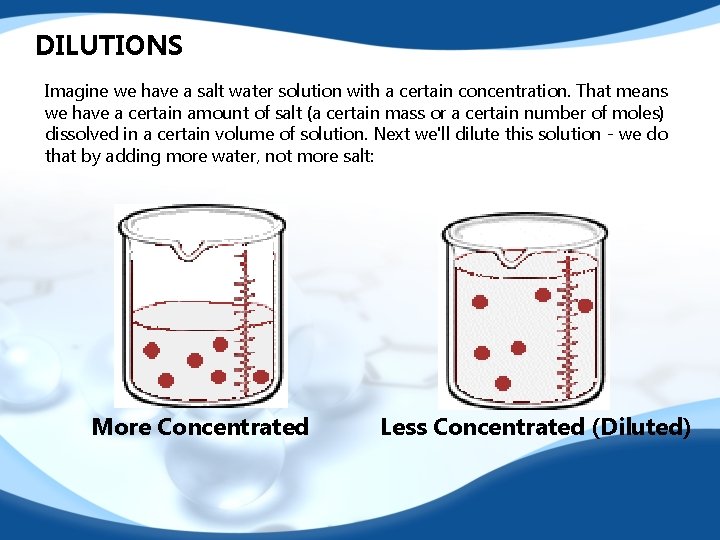
DILUTIONS Imagine we have a salt water solution with a certain concentration. That means we have a certain amount of salt (a certain mass or a certain number of moles) dissolved in a certain volume of solution. Next we'll dilute this solution - we do that by adding more water, not more salt: More Concentrated Less Concentrated (Diluted)

V 1 M 1 = V 2 M 2 What volume of concentrated sulfuric acid, 18. 0 M, is required to prepare 5. 00 L of 0. 150 M solution by dilution with water?

1. A chemist starts with 50. 0 m. L of a 0. 40 M Na. Cl solution and dilutes it to 1000. m. L. What is the concentration of Na. Cl in the new solution?

2. A chemist wants to make 500. m. L of 0. 050 M HCl by diluting a 6. 0 M HCl solution. How much of that solution should be used?

3. How much 2. 0 M Na. Cl solution would you need to make 250 m. L of 0. 15 M Na. Cl solution?