Section 1 The Nature of Energy Energy is







- Slides: 23


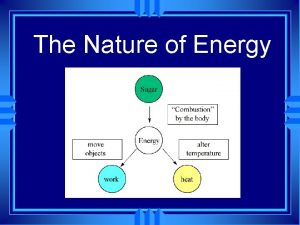
Section 1: The Nature of Energy • Energy is the ability to cause change. • There are many different forms of energy: – chemical – thermal – kinetic – potential – electrical

Potential and Kinetic Energy

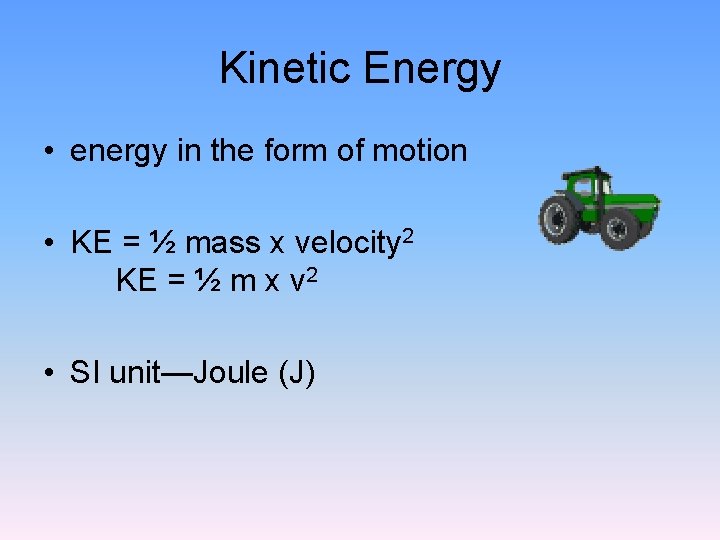
Kinetic Energy • energy in the form of motion • KE = ½ mass x velocity 2 KE = ½ m x v 2 • SI unit—Joule (J)

Potential Energy • stored energy due to position • types of potential energy – elastic: energy stored by something that can stretch or compress (ex. rubber band, spring) – chemical: energy stored in chemical bonds (ex. gasoline, food) – gravitational: energy stored by objects that are above Earth’s surface (ex. apple or leaf in tree)

Gravitational Potential Energy (GPE) • GPE = mass x 9. 8 m/s 2 x height • SI unit—Joule (J) • Bjorn is holding a tennis ball outside a 2 nd story window (3. 5 m from the ground) and Billie Jean is holding one outside a 3 rd story window (6. 25 m from the ground). How much more GPE does Billie Jean’s tennis ball have? (Each tennis ball has a mass of 0. 06 kg).

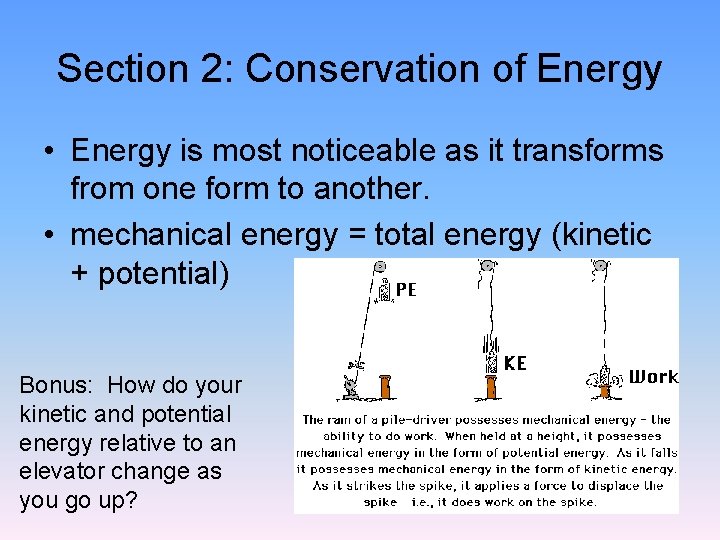
Section 2: Conservation of Energy • Energy is most noticeable as it transforms from one form to another. • mechanical energy = total energy (kinetic + potential) Bonus: How do your kinetic and potential energy relative to an elevator change as you go up?

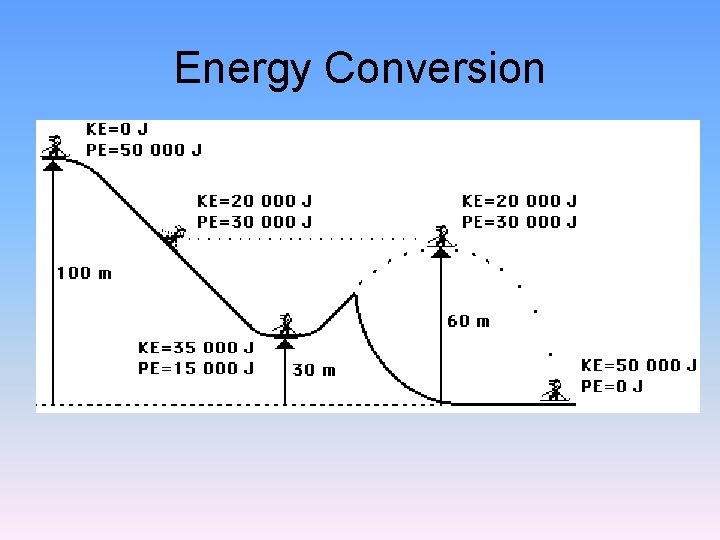
Energy Conversion

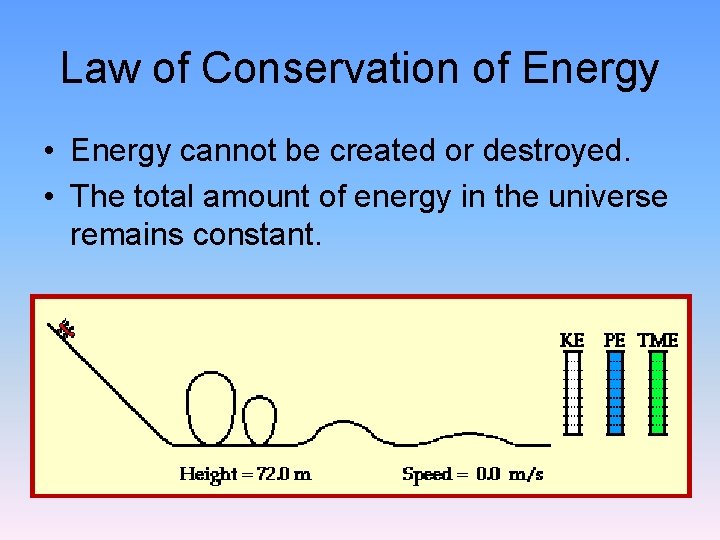
Law of Conservation of Energy • Energy cannot be created or destroyed. • The total amount of energy in the universe remains constant.

Work (Chapter 5 -1) • Work is the transfer of energy that occurs when a force makes an object move. • Two conditions must be met for work to be done on an object: – the object has to move – the movement must be in the direction of the force

Work and Energy • When work is done, a transfer of energy always occurs.

Calculating Work • Work = force x distance W=Fxd • SI unit: Joules (J) • One joule is about the amount of work required to lift a baseball a vertical distance of 0. 7 m.

Example Problems • You move a 75 kg refrigerator 35 m. This requires a force of 90 N. How much work was done while moving the refrigerator? – 3150 J • When you and a friend move a 45 kg couch to another room, you exert a force of 75 N over 5 m. How much work did you do? – 375 J

Bonus • Suppose you used a force of 50 N to shoot an arrow, and the arrow flew 25 meters. As you shot the arrow, the bow string moved the arrow 1 m. Did you do 1250 J of work or 50 J of work? Explain. – You did 50 J of work, because after the arrow left the bow, it was flying loose in the air and was not experiencing any force from you.

Power • Power is the amount of work done in a certain amount of time (the rate at which work is done). • Power = work / time P=W/t • SI unit: watts • Example: How much power is required to push a car for 10 s if the amount of work done during that time is 5, 500 J? – 550 W

Temperature and Heat (Chapter 6) • All matter is made up of tiny particles in constant motion, meaning that they have kinetic energy. • temperature: average kinetic energy of the particles

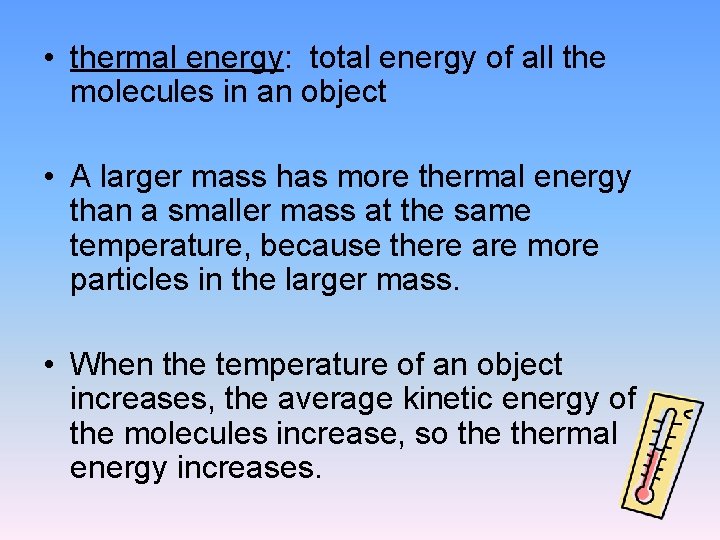
• thermal energy: total energy of all the molecules in an object • A larger mass has more thermal energy than a smaller mass at the same temperature, because there are more particles in the larger mass. • When the temperature of an object increases, the average kinetic energy of the molecules increase, so thermal energy increases.

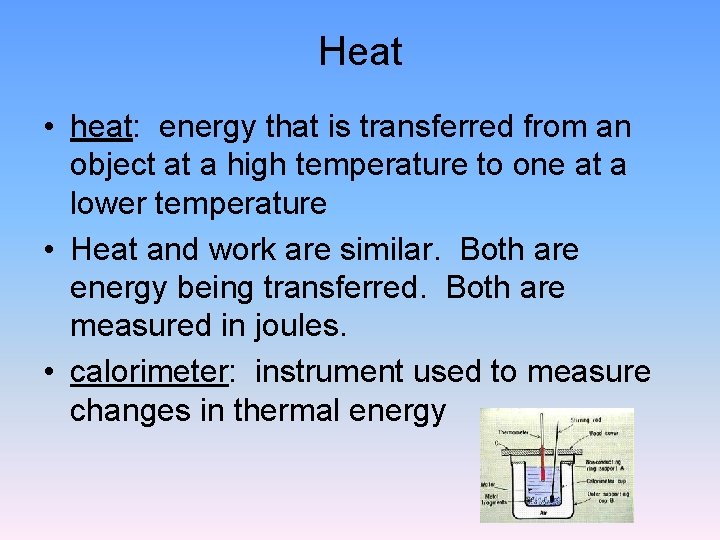
Heat • heat: energy that is transferred from an object at a high temperature to one at a lower temperature • Heat and work are similar. Both are energy being transferred. Both are measured in joules. • calorimeter: instrument used to measure changes in thermal energy

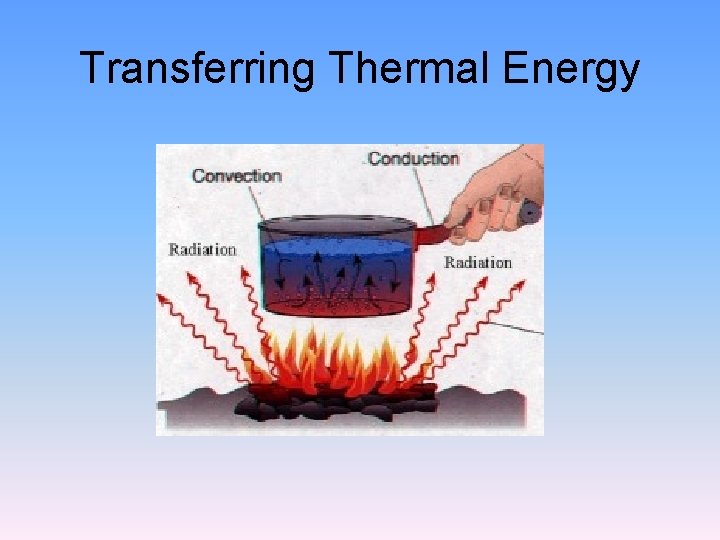
Transferring Thermal Energy

Conduction • conduction: transfer of energy through matter by the direct contact of particles • Conduction occurs because of the collisions between particles. • insulators: material that doesn’t allow heat to flow through it easily

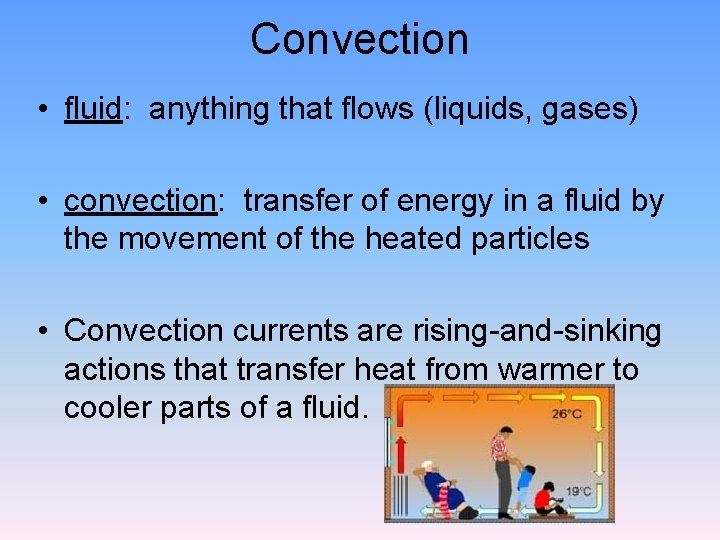
Convection • fluid: anything that flows (liquids, gases) • convection: transfer of energy in a fluid by the movement of the heated particles • Convection currents are rising-and-sinking actions that transfer heat from warmer to cooler parts of a fluid.

Radiation • radiation: transfer of energy by electromagnetic waves • Electromagnetic waves can travel through space even when no matter is present. • When radiation strikes a material, some of the energy is absorbed, some is reflected and some may be transmitted through the material.

 Section 1 what is energy answer key
Section 1 what is energy answer key Chapter 4 section 1 the nature of energy worksheet answers
Chapter 4 section 1 the nature of energy worksheet answers The nature of energy section 1
The nature of energy section 1 Section 1 work and machines section 2 describing energy
Section 1 work and machines section 2 describing energy Nature and nature's law lay hid in night meaning
Nature and nature's law lay hid in night meaning Nature nature controversy
Nature nature controversy Chapter 5 thermal energy answer key
Chapter 5 thermal energy answer key Chapter 8 section 1 how organisms obtain energy answer key
Chapter 8 section 1 how organisms obtain energy answer key Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Glasgow thang điểm
Glasgow thang điểm Hát lên người ơi
Hát lên người ơi Môn thể thao bắt đầu bằng từ đua
Môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới