RNA PROCESSING AND RNPs RNA Processing Very few

















- Slides: 67

RNA PROCESSING AND RNPs

RNA Processing Ø Very few RNA molecules are transcribed directly into the final mature RNA. Ø Most newly transcribed RNA molecules (primary transcripts) undergo various alterations to yield the mature product Ø RNA processing is the collective term used to describe the molecular events allowing the primary transcripts to become the mature RNA.

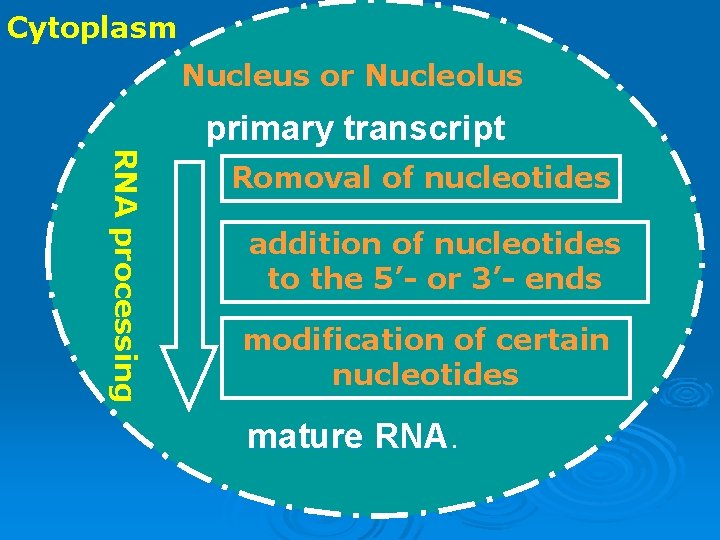
Cytoplasm Nucleus or Nucleolus RNA processing primary transcript Romoval of nucleotides addition of nucleotides to the 5’- or 3’- ends modification of certain nucleotides mature RNA.

(1) Removal of nucleotides by both endonucleases and exonucleases Ø endonucleases to cut at specific sites within a precursor RNA Ø exonucleases to trim the ends of a precursor RNA Ø This general process is seen in prokaryotes and eukaryotes for all types of RNA

(2) Addition of nucleotides to 5’-or 3’ends of the primary transcripts or their cleavage products. Add a cap and a poly(A) tail to pre-m. RNA

(3) Modification of certain nucleotides on either the base or the sugar moiety. –Add a methyl group to 2’-OH of ribose in m. RNA (A) and r. RNA –Extensive changes of bases in t. RNA

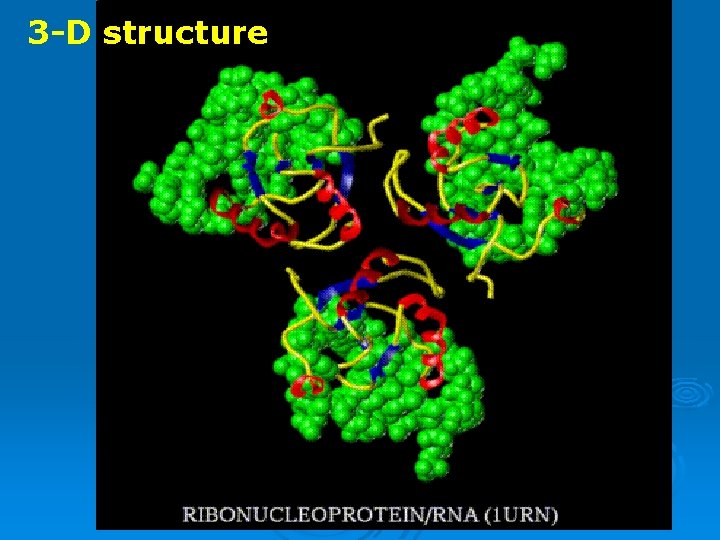
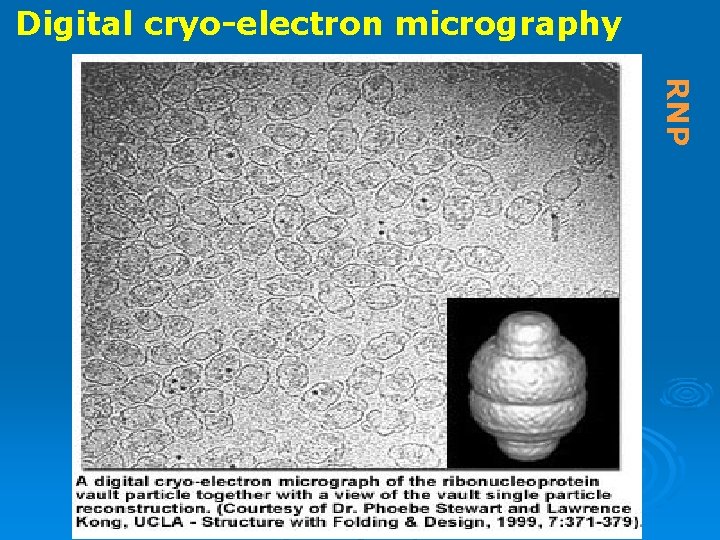
RNPs Ribonucleoproteins = RNA protein complexs Ø The RNA molecules in cells usually exist complexed with proteins Ø specific proteins attach to specific RNAs Ø Ribosomes are the largest and most complex RNPs

3 -D structure

Digital cryo-electron micrography RNP

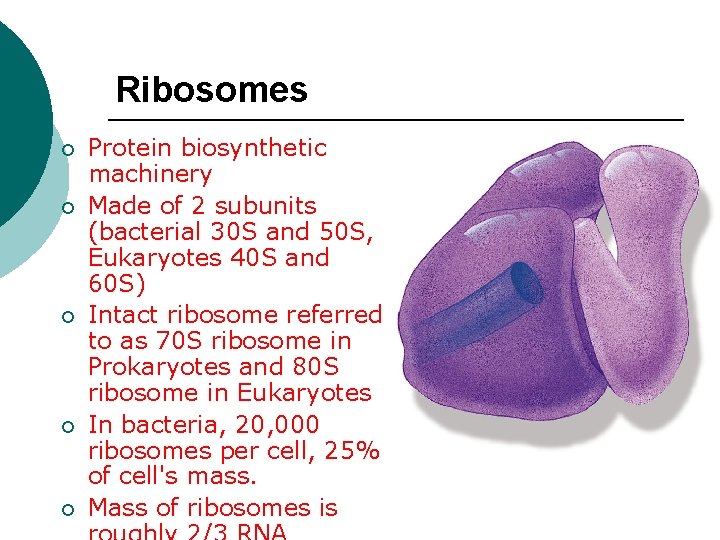
Ribosomes ¡ ¡ ¡ Protein biosynthetic machinery Made of 2 subunits (bacterial 30 S and 50 S, Eukaryotes 40 S and 60 S) Intact ribosome referred to as 70 S ribosome in Prokaryotes and 80 S ribosome in Eukaryotes In bacteria, 20, 000 ribosomes per cell, 25% of cell's mass. Mass of ribosomes is

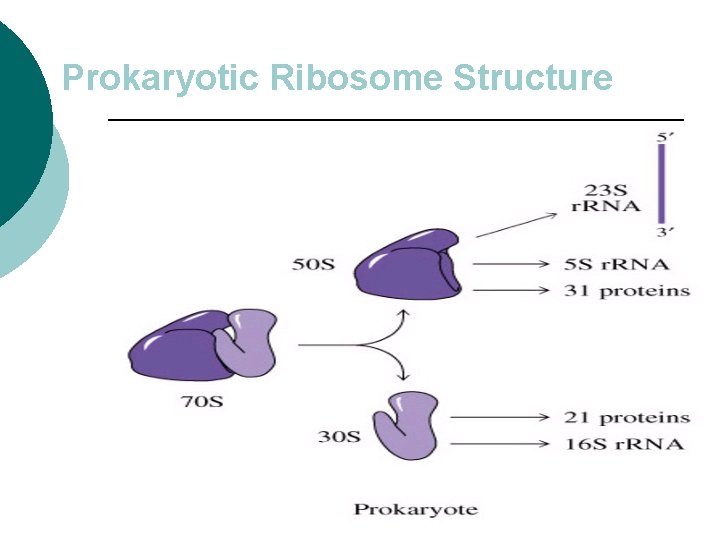
Prokaryotic Ribosome Structure

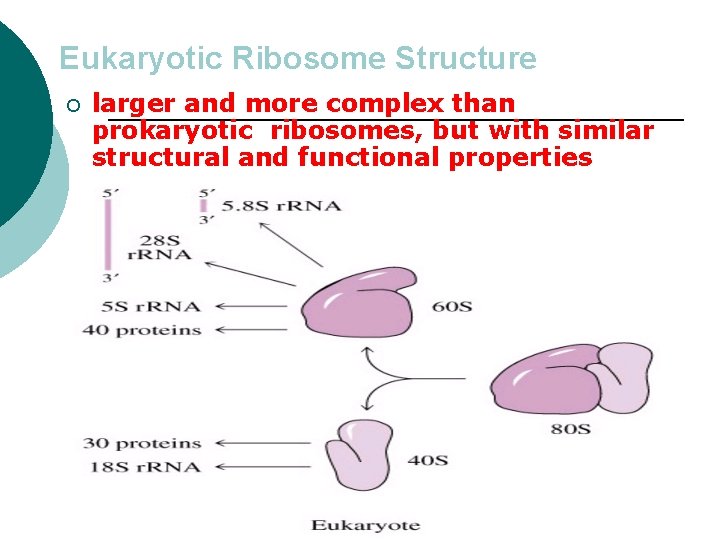
Eukaryotic Ribosome Structure ¡ larger and more complex than prokaryotic ribosomes, but with similar structural and functional properties

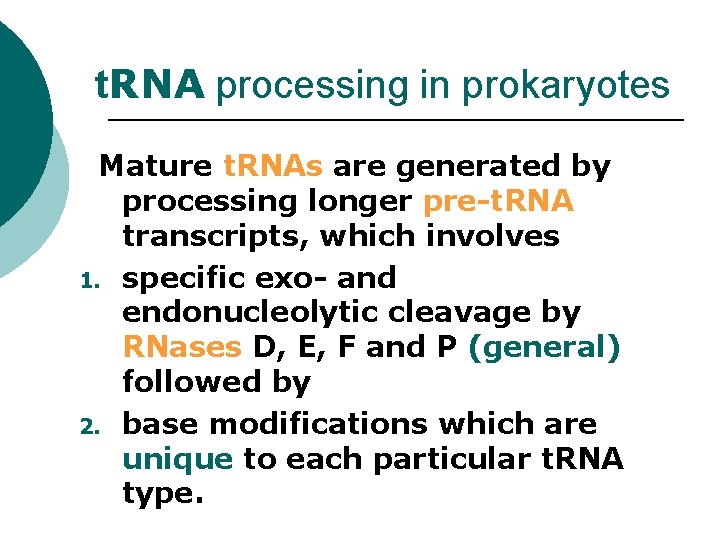
t. RNA PROCESSING, RNASE P RIBOZYMES AND o t. RNA processing in prokaryotes o t. RNA processing in eukaryotes o RNase P o Ribozymes

t. RNA 3 -D structure

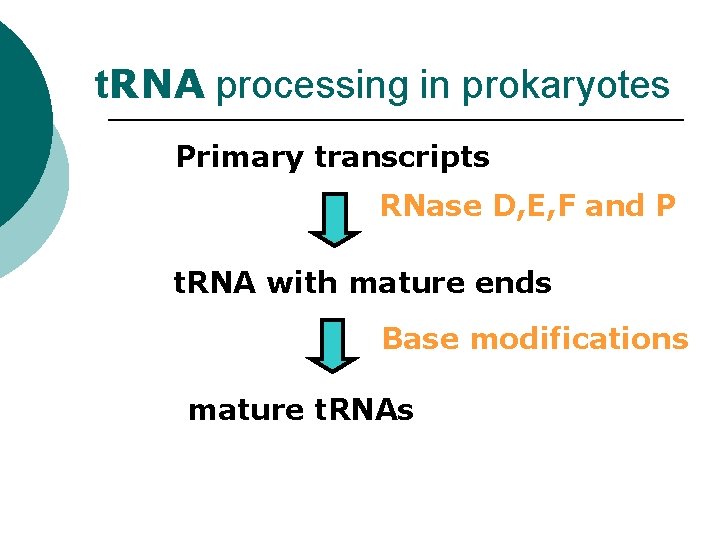
t. RNA processing in prokaryotes Mature t. RNAs are generated by processing longer pre-t. RNA transcripts, which involves 1. specific exo- and endonucleolytic cleavage by RNases D, E, F and P (general) followed by 2. base modifications which are unique to each particular t. RNA type.

t. RNA processing in prokaryotes Primary transcripts RNase D, E, F and P t. RNA with mature ends Base modifications mature t. RNAs

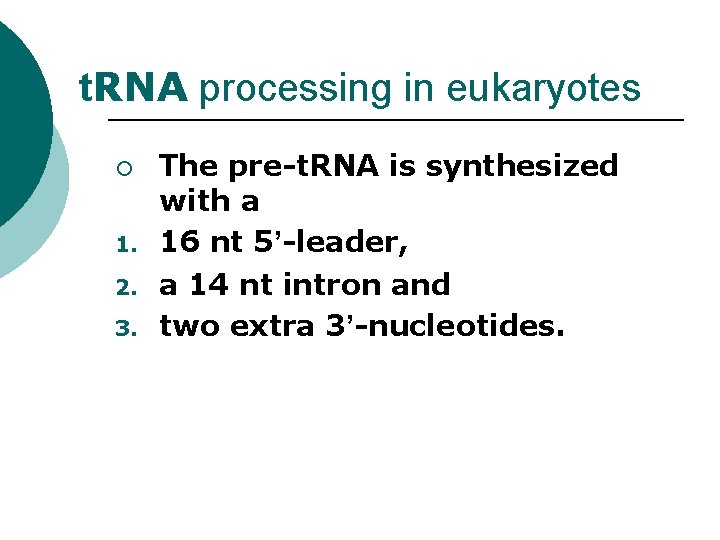
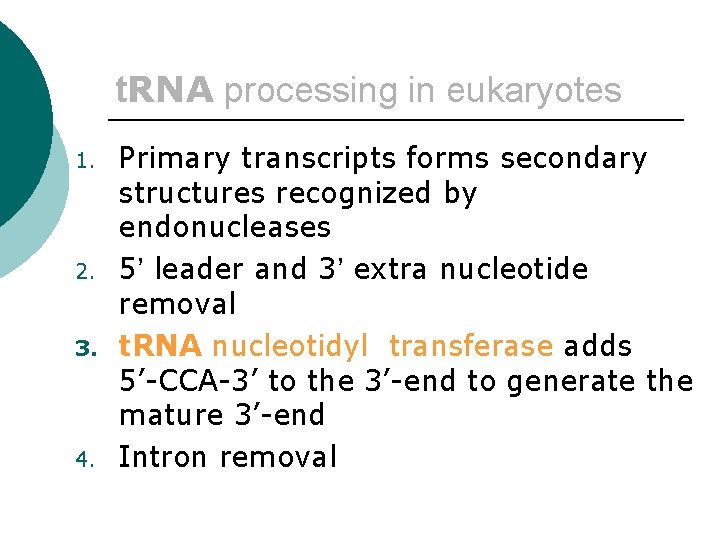
t. RNA processing in eukaryotes ¡ 1. 2. 3. The pre-t. RNA is synthesized with a 16 nt 5’-leader, a 14 nt intron and two extra 3’-nucleotides.


t. RNA processing in eukaryotes 1. 2. 3. 4. Primary transcripts forms secondary structures recognized by endonucleases 5’ leader and 3’ extra nucleotide removal t. RNA nucleotidyl transferase adds 5’-CCA-3’ to the 3’-end to generate the mature 3’-end Intron removal

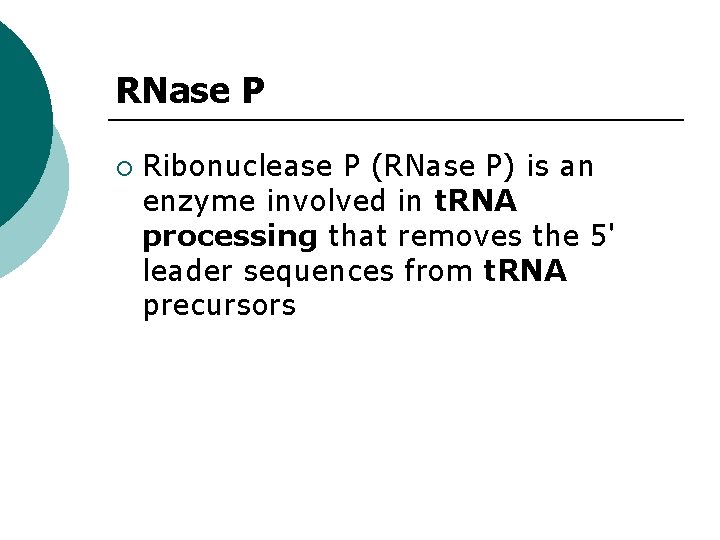
RNase P ¡ Ribonuclease P (RNase P) is an enzyme involved in t. RNA processing that removes the 5' leader sequences from t. RNA precursors

RNase P ¡ RNase P enzymes are found in both prokaryotes and eukaryotes, being located in the nucleus of the latter where they are therefore small nuclear RNPs (sn. RNPs)

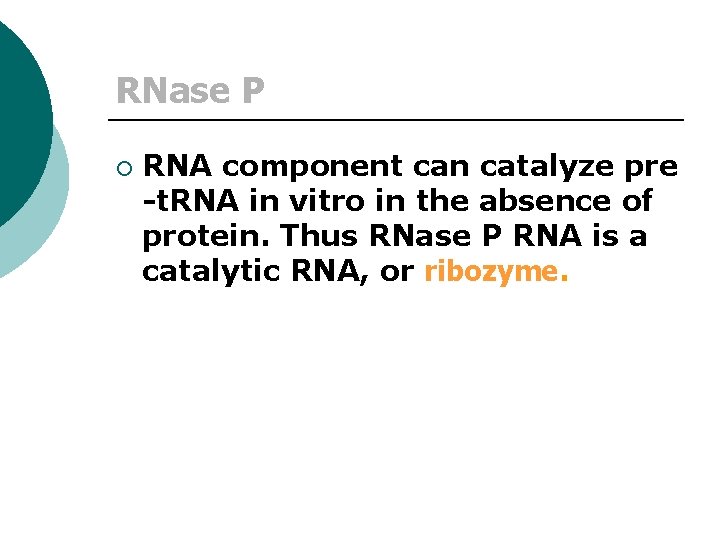
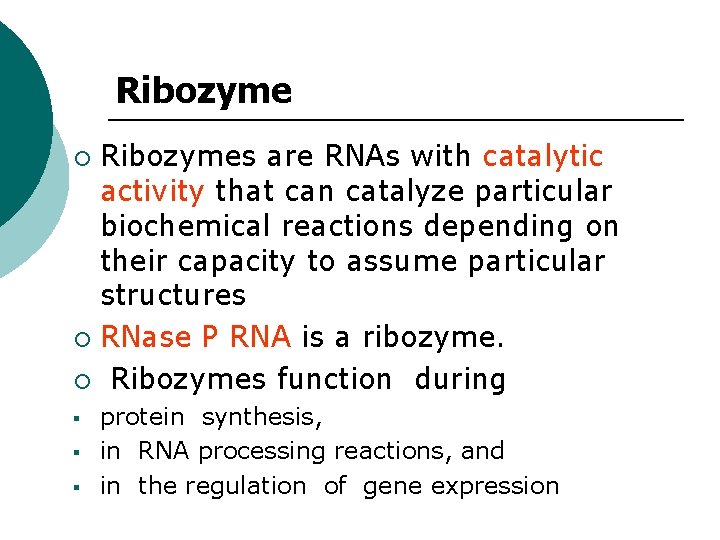
RNase P ¡ RNA component can catalyze pre -t. RNA in vitro in the absence of protein. Thus RNase P RNA is a catalytic RNA, or ribozyme.

Ribozymes are RNAs with catalytic activity that can catalyze particular biochemical reactions depending on their capacity to assume particular structures ¡ RNase P RNA is a ribozyme. ¡ Ribozymes function during ¡ § § § protein synthesis, in RNA processing reactions, and in the regulation of gene expression

Ribozyme ¡ Self-splicing introns: the intervening RNA that catalyze the splicing of themselves from their precursor RNA, and the joining of the exon sequences

Ribozyme ¡ Self-cleaving RNA encoded by viral genome to resolve the concatameric molecules of the viral genomic RNA produced. These molecules are able to fold up in such a way as to selfcleave themselves into monomeric.

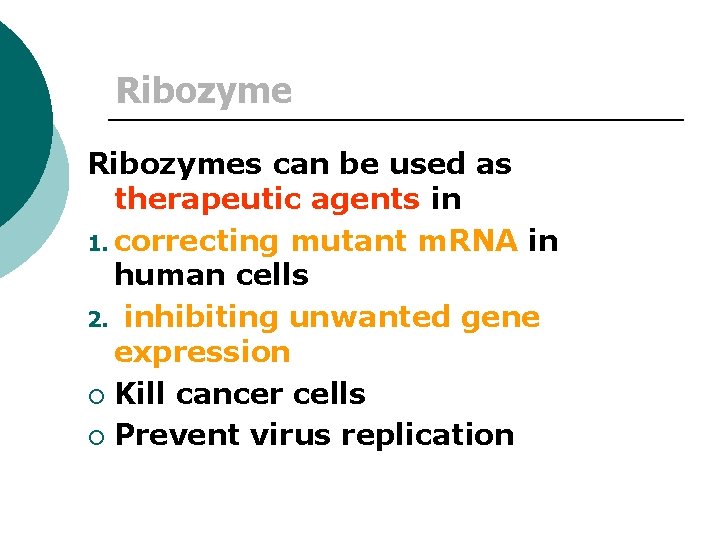
Ribozymes can be used as therapeutic agents in 1. correcting mutant m. RNA in human cells 2. inhibiting unwanted gene expression ¡ Kill cancer cells ¡ Prevent virus replication

The Power of RNA interference RNA Interference (RNAi) is able to block selective m. RNA LOSS OF FUNCTION Easy in yeast Difficult in mammals

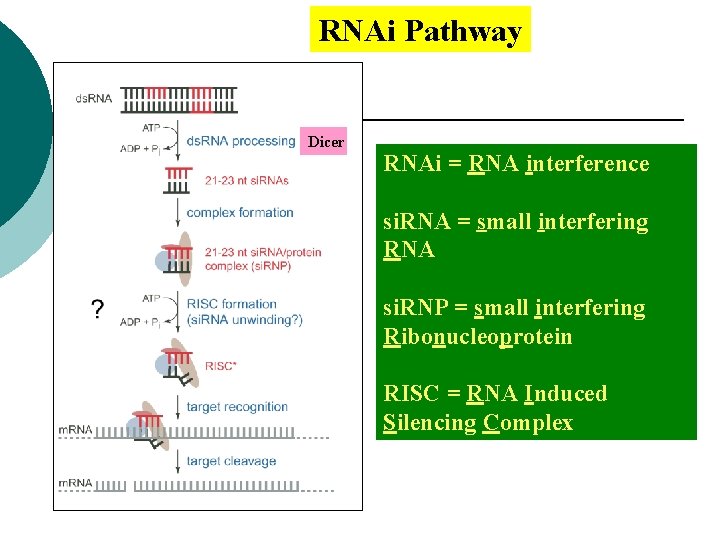
RNAi Pathway Dicer RNAi = RNA interference si. RNA = small interfering RNA si. RNP = small interfering Ribonucleoprotein RISC = RNA Induced Silencing Complex

Inhibition the replication of SARS virus by RNA interference

m. RNA PROCESSING, hn. RNPs sn. RNPs o o o o AND Processing of m. RNA hn. RNP sn. RNP particles 5’Capping 3’Cleavage and polyadenylation Splicing Pre-m. RNA methylation

Processing of m. RNA There is essentially no processing of prokaryotic m. RNA, it can start to be translated before it has finished being transcribed. ¡ Prokaryotic m. RNA is degraded rapidly from the 5’ end ¡

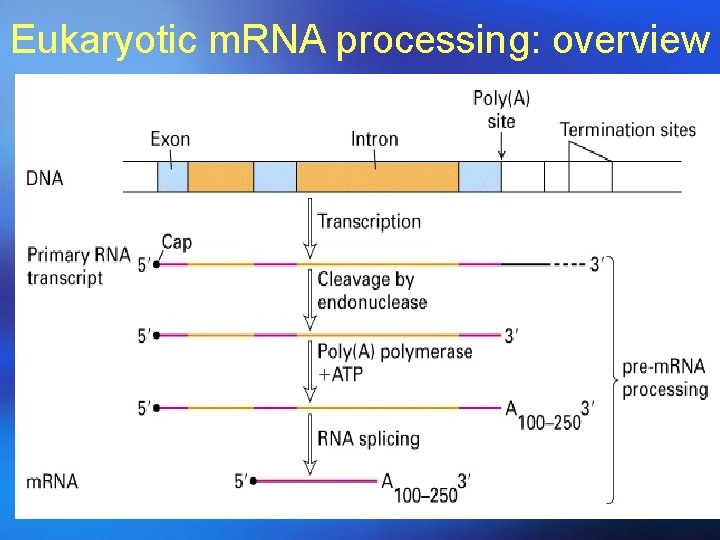
Processing of m. RNA in eukaryotes In eukaryotes, m. RNA is synthesized by RNA Pol II as longer precursors (pre-m. RNA), the population of different RNA Pol II transcripts are called heterogeneous nuclear RNA (hn. RNA). ¡ Among hn. RNA, those processed to give mature m. RNAs are called prem. RNAs ¡

Processing of m. RNA in eukaryotes ¡Pre-m. RNA molecules are processed to mature m. RNAs by 5’-capping, 3’cleavage and polyadenylation, splicing and methylation.

Eukaryotic m. RNA processing: overview

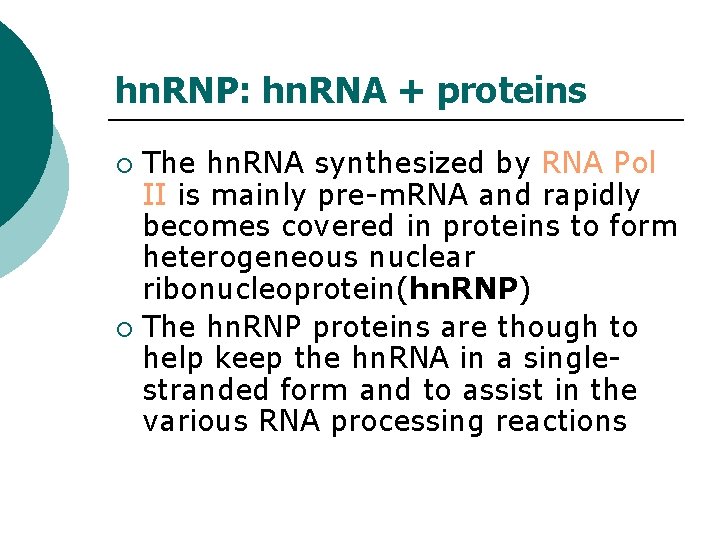
hn. RNP: hn. RNA + proteins The hn. RNA synthesized by RNA Pol II is mainly pre-m. RNA and rapidly becomes covered in proteins to form heterogeneous nuclear ribonucleoprotein(hn. RNP) ¡ The hn. RNP proteins are though to help keep the hn. RNA in a singlestranded form and to assist in the various RNA processing reactions ¡

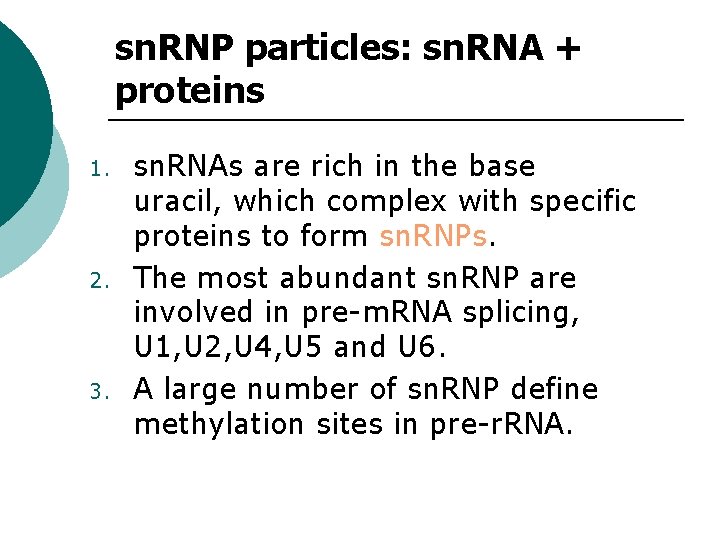
sn. RNP particles: sn. RNA + proteins 1. 2. 3. sn. RNAs are rich in the base uracil, which complex with specific proteins to form sn. RNPs. The most abundant sn. RNP are involved in pre-m. RNA splicing, U 1, U 2, U 4, U 5 and U 6. A large number of sn. RNP define methylation sites in pre-r. RNA.

sn. RNP particles They are synthesized in the nucleus by RNA Pol II and have a normal 5’-cap. ¡ They are exported to the cytoplasm where they associate with the common core proteins and with other specific proteins. ¡ Their 5’-cap gains two methyl groups and they are then imported back into the nucleus where they function in splicing. ¡


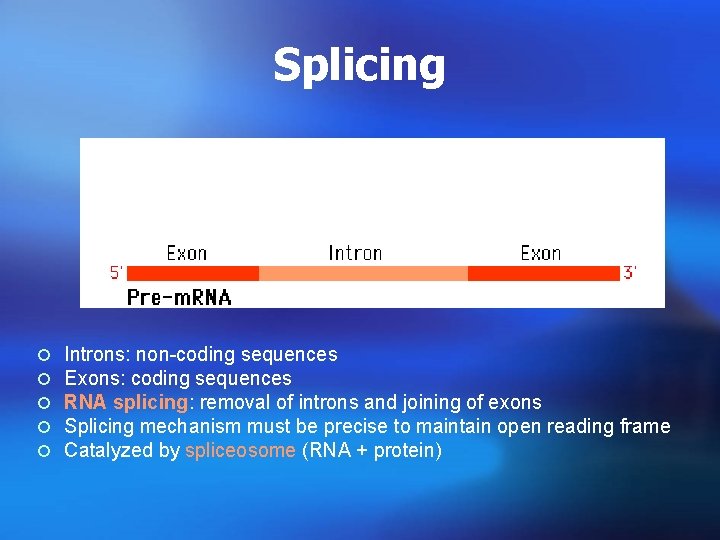
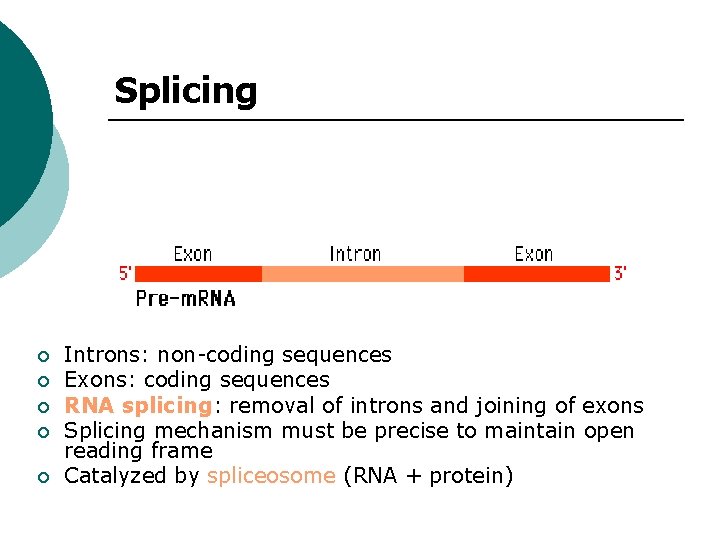
Splicing ¡ ¡ ¡ Introns: non-coding sequences Exons: coding sequences RNA splicing: removal of introns and joining of exons Splicing mechanism must be precise to maintain open reading frame Catalyzed by spliceosome (RNA + protein)

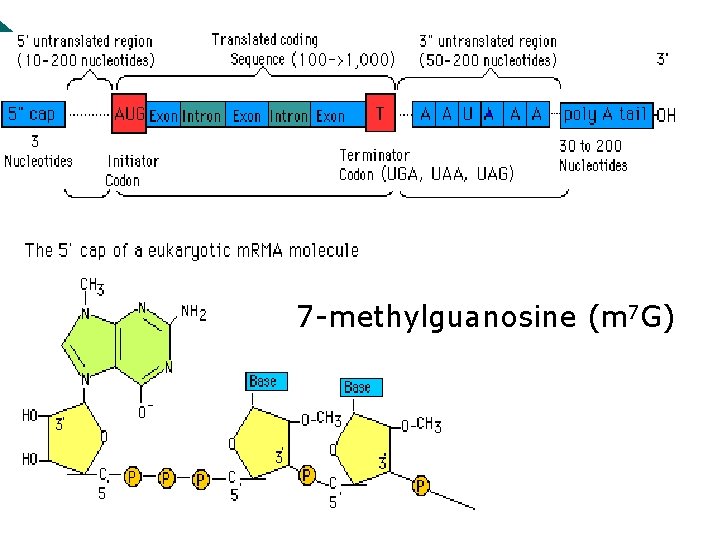
5’ Capping ¡ ¡ Very soon after RNA Pol II starts making a transcript, and before the RNA chain is more than 20 -30 nt long, the 5’-end is chemically modified. 7 -methylguanosine is covalently to the 5´ end of pre-m. RNA. Linked 5´ Occurs shortly after initiation

7 -methylguanosine (m 7 G)


Function of 5´ cap Protection from degradation ¡ Increasing translational efficiency ¡ Transport to cytoplasm ¡ Splicing of first exon ¡

3’ Cleavage and polyadenylation ¡ In most pre-m. RNAs, the mature 3’-end of the molecule is generated by cleavage followed by the addition of a run, or tail, of A residues which is called the poly(A) tail.

3’ Cleavage and polyadenylation RNA polymerase II does not usually terminate at distinct site ¡ Pre-m. RNA is cleaved ~20 nucleotides downstream of polyadenylation signal (AAUAAA) ¡ ~250 AMPs are then added to the 3´ end ¡ Almost all m. RNAs have poly(A) tail ¡

Function of poly(A) tail Increasing m. RNA stability ¡ Increasing translational efficiency ¡ Splicing of last intron ¡

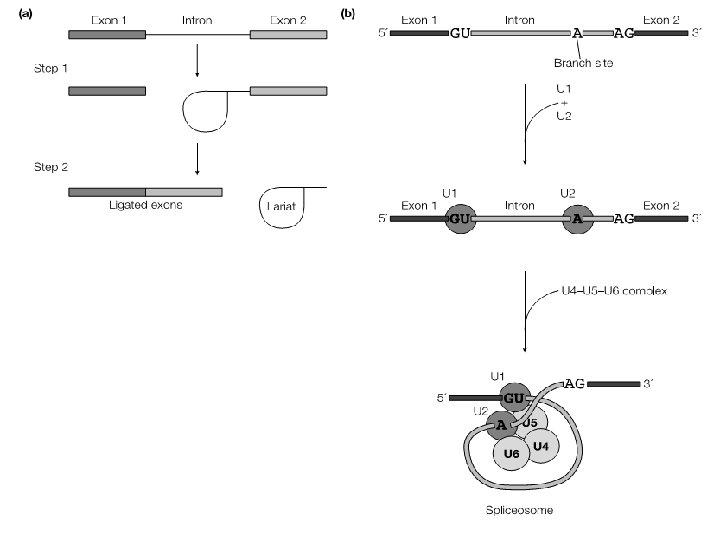
Splicing the process of cutting the pre-m. RNA to remove the introns and joining together of the exons is called splicing. ¡ it takes place in the nucleus before the mature m. RNA can be exported to the cytoplasm. ¡

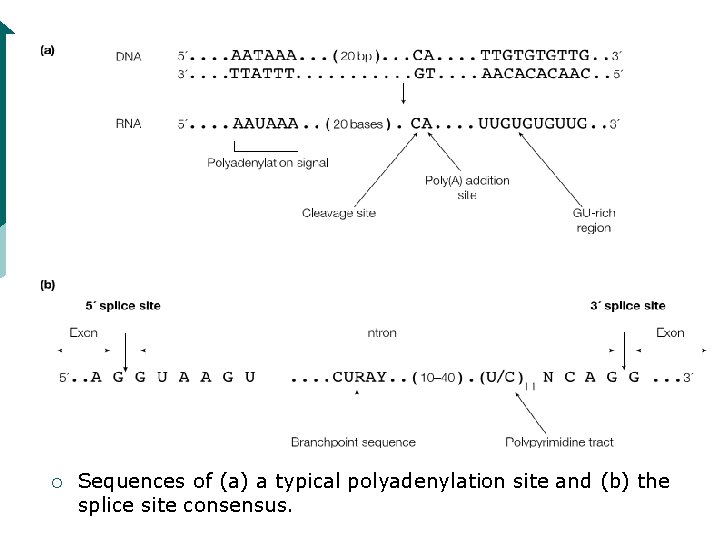
Splicing ¡ Splicing requires a set of specific sequences to be present. The 5’-end of almost all introns has the sequence 5’-GU-3’ and the 3’-end is usually 5’-AG-3’. The AG at the 3’end is preceded by a pyrimidine-rich sequence called the polypyrimidine tract

¡ Sequences of (a) a typical polyadenylation site and (b) the splice site consensus.

Spliceosome Catalyzes pre-m. RNA splicing in nucleus ¡ Composed of five small nuclear RNAs (sn. RNAs) and associated proteins (sn. RNPs) assembled on the prem. RNA ¡ Splicing reaction is catalyzed by RNA ¡


Splicing cycle

Splicing ¡ ¡ ¡ Introns: non-coding sequences Exons: coding sequences RNA splicing: removal of introns and joining of exons Splicing mechanism must be precise to maintain open reading frame Catalyzed by spliceosome (RNA + protein)

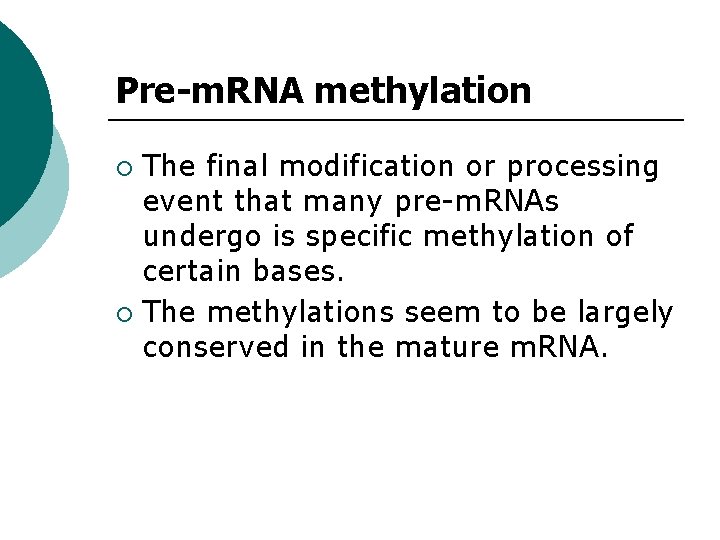
Pre-m. RNA methylation The final modification or processing event that many pre-m. RNAs undergo is specific methylation of certain bases. ¡ The methylations seem to be largely conserved in the mature m. RNA. ¡

ALTERNATIVE m. RNA PROCESSING o Alternative processing o Alternative poly(A) sites o Alternative splicing o RNA editing

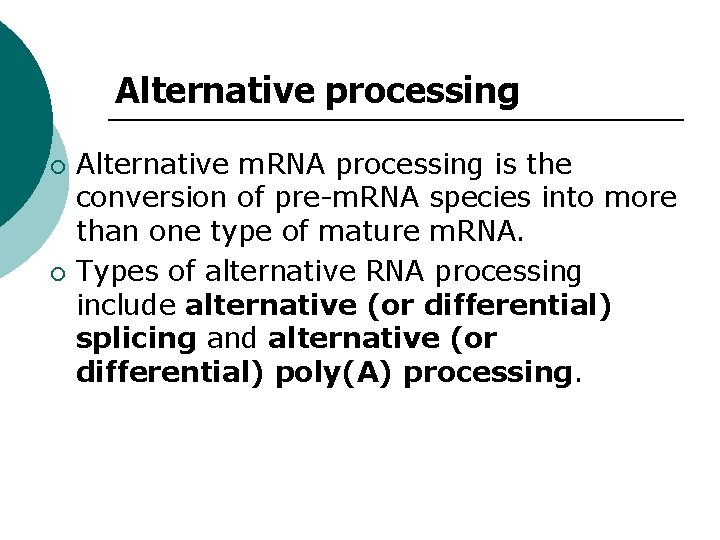
Alternative processing ¡ ¡ Alternative m. RNA processing is the conversion of pre-m. RNA species into more than one type of mature m. RNA. Types of alternative RNA processing include alternative (or differential) splicing and alternative (or differential) poly(A) processing.

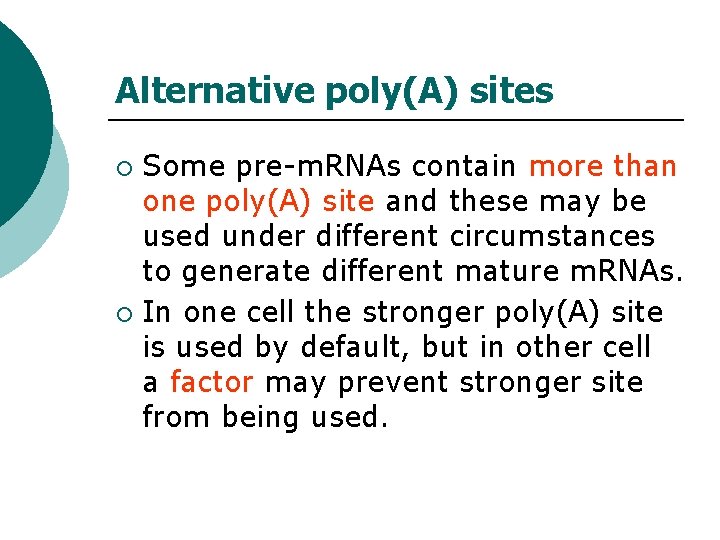
Alternative poly(A) sites Some pre-m. RNAs contain more than one poly(A) site and these may be used under different circumstances to generate different mature m. RNAs. ¡ In one cell the stronger poly(A) site is used by default, but in other cell a factor may prevent stronger site from being used. ¡

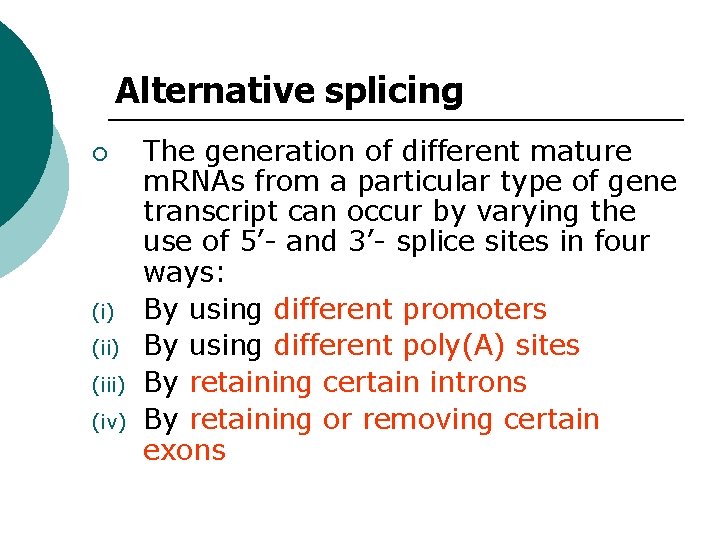
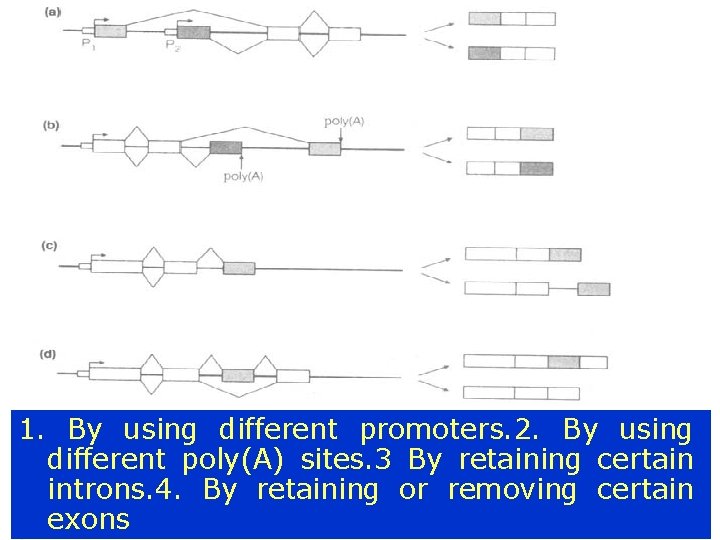
Alternative splicing ¡ (i) (iii) (iv) The generation of different mature m. RNAs from a particular type of gene transcript can occur by varying the use of 5’- and 3’- splice sites in four ways: By using different promoters By using different poly(A) sites By retaining certain introns By retaining or removing certain exons

Alternative splicing 1. By using different promoters. 2. By using different poly(A) sites. 3 By retaining certain introns. 4. By retaining or removing certain exons

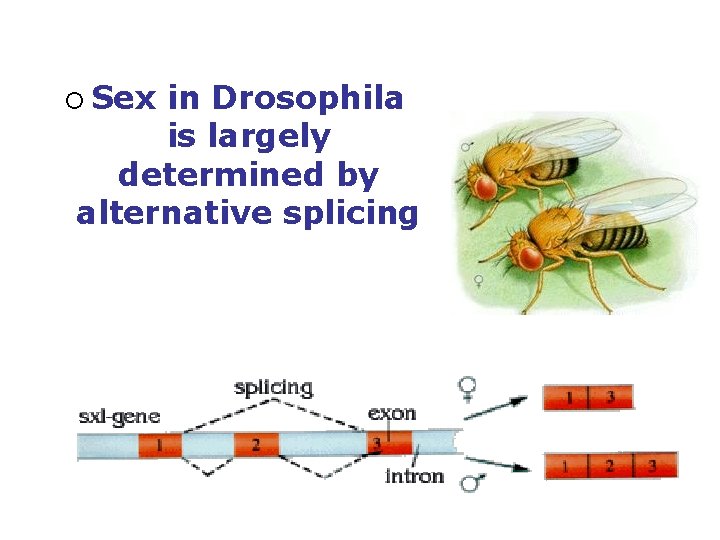
¡ Sex in Drosophila is largely determined by alternative splicing

Alternative splicing • the potential for an increase in phenotypic diversity without increasing the overall number of genes. Is achieved by altering the pattern of exons that are spliced together, • different proteins can arise from the processed m. RNA from a single gene.

Alternative splicing • Alternative splicing can occur either at specific developmental stages or in different cell types. • the calcitonin gene yields an m. RNA that synthesizes calcitonin (thyroid) or calcitonin gene– related peptide (CGRP, brain): 2 proteins with distinctly different functions. • the α-tropomyosin m. RNA have at least 8 different alternatively spliced α-tropomyosin m. RNAs.


Alternative splicing Many defects in the β-globin genes are known to exist leading to β-thalassemias. Some of these defects are caused by mutations in the sequences of the m. RNA required for intron recognition and, therefore, result in abnormal processing of the β-globin primary transcript.

RNA editing AN unusual form of RNA processing in which the sequence of the primary transcript is altered is called RNA editing. ¡ Changing RNA sequence (after transcription) ¡ Two types ¡ l l Base modification (A or C deamination) Base (U) insertion and deletion


End Of The lecture Thanks!