Recapitulate WaveParticle duality implies that position and momentum













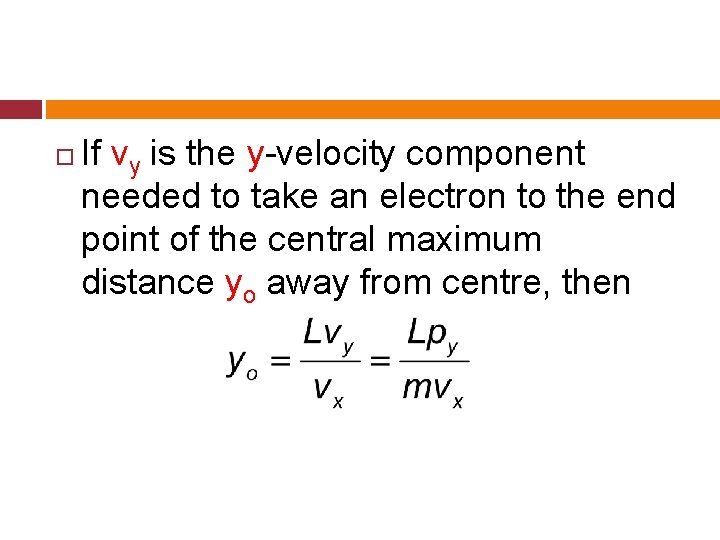






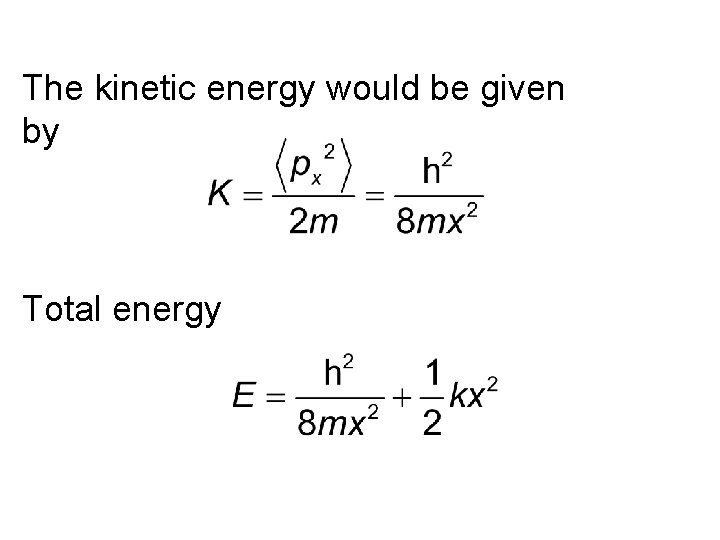
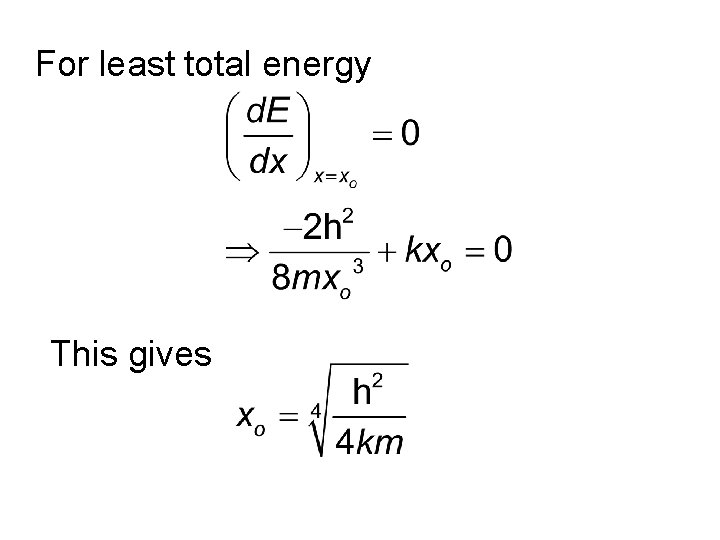
- Slides: 38

Recapitulate Wave-Particle duality implies that position and momentum of a particle can not be simultaneously determined to perfect precision. This forms the basis of Uncertainty Principle.

Young’s Double Slit Experiment Observation of interference pattern with the electrons implies that number of electrons reaching a point on the screen can not be the sum of electrons reaching from either of the slits when only one of the slit is open.

Interference pattern is seen even if one electron passes through the slits at a time, without any possibility of electrostatic or any other interaction between electrons.

How Electrons Reach the Screen? If we try to find out from which slit the electrons go, we shall find that indeed the electrons go trough one of the two slits. But in finding out this we shall perturb the experiment enough so as to loose the interference pattern.

In case we do not try to find how electrons reach the screen, the interference pattern remains in tact. Does uncertainty means taking a backwards step in Science?

1989 experiment by Tonomura http: //www. hitachi. com/rd/research/em/ doubleslit. html

Acknowledgements Prof. Claude Cohen-Tannoudji for sending me this link and another film on Ne atoms, which show a similar interference pattern.


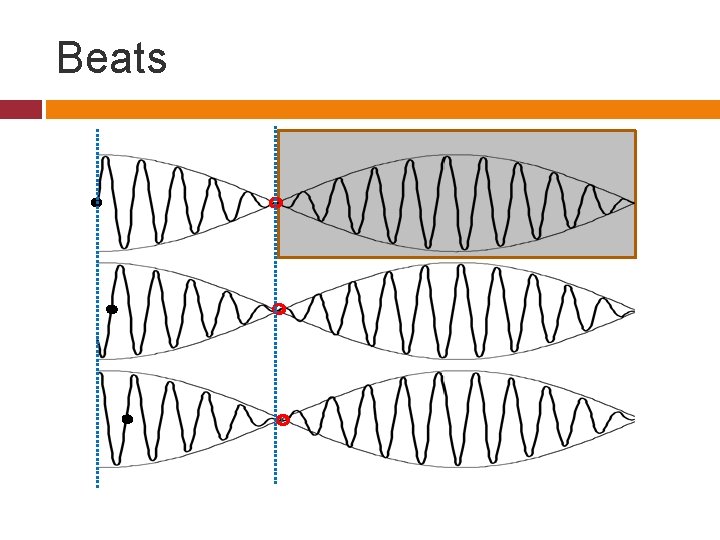
Uncertainty: (Wave Packet Approach) Go back to old beats’ problem. Assume that one modulation of the wave packet can be created by mixing waves of not just two wave lengths but all the wave lengths between k and k+Δk.

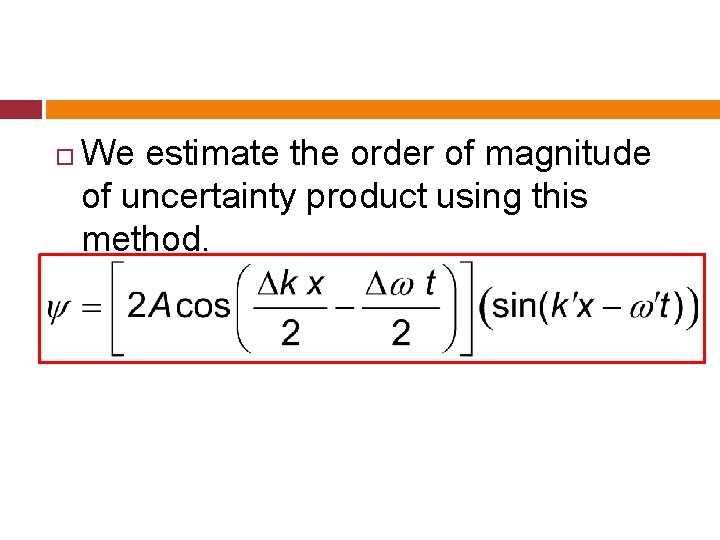
We estimate the order of magnitude of uncertainty product using this method.

Beats

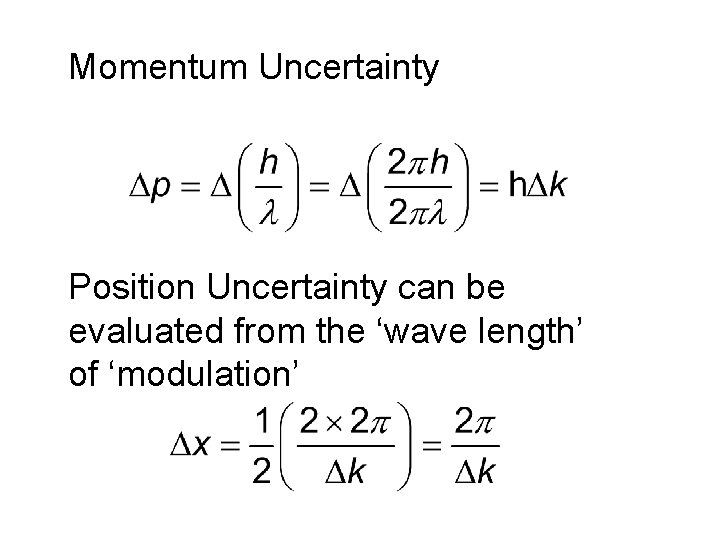
Momentum Uncertainty Position Uncertainty can be evaluated from the ‘wave length’ of ‘modulation’

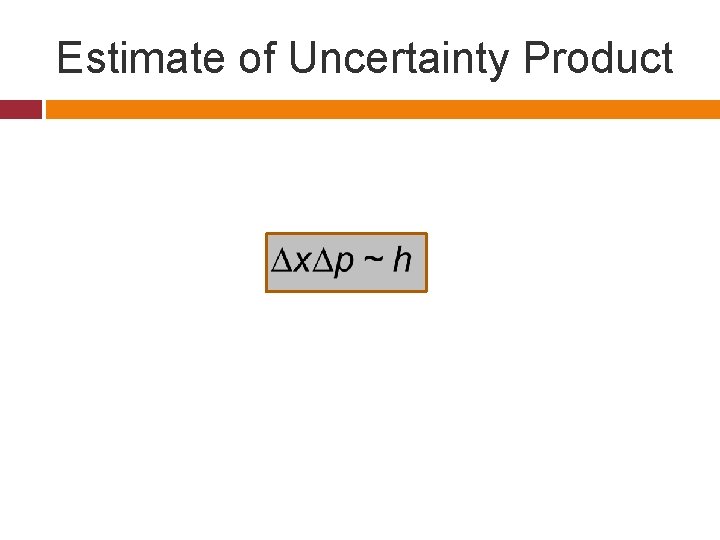
Estimate of Uncertainty Product

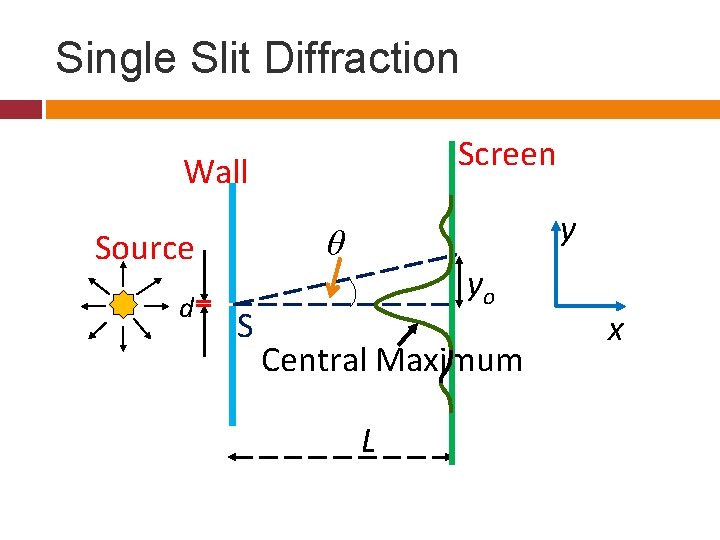
Single Slit Diffraction Screen Wall θ Source d y S yo Central Maximum L x

Features The angular position where we see a minima is given by the following.

Consequences 1. The smaller is the slit width, larger is the width of the central maximum. 2. The experiment can be understood well by the wave theory. 3. Now let us bring the particle nature assuming that the experiment is being performed with particles.

Particles Approaching Assume the source is infinite distance away. Therefore, electrons move in x-direction. Hence we are nor sure of the yposition of the particle at all. What happens when the particle passes through the slit?

Particles Passing through the Slit Δy is suddenly made finite. Hence Δpy can also not be zero any more. Hence the particles would pick up a y -component of momentum in a totally unpredictable and uncontrolled manner.

Smaller is the slit width, larger is the uncertainty in momentum component. Can’t we find out the momentum after an electron reaches the screen? Newton’s law? ?

Order of Uncertainty: (Wave Particle Duality Approach) Let us use the wave formula to predict the order of uncertainty product. Let us assume that the electrons land themselves only in the central maximum of the diffraction pattern.

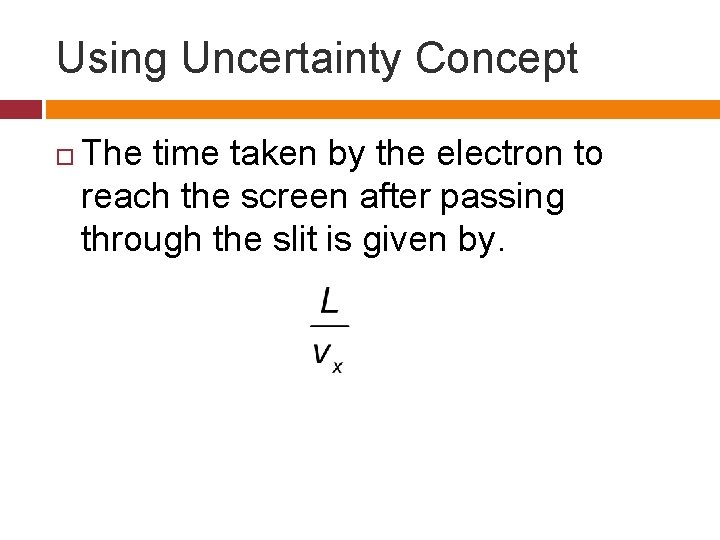
Using Uncertainty Concept The time taken by the electron to reach the screen after passing through the slit is given by.

During this time the electron also gets displaced along y-direction due to the random velocity acquired after passing through the slit, due to uncertainty principle.
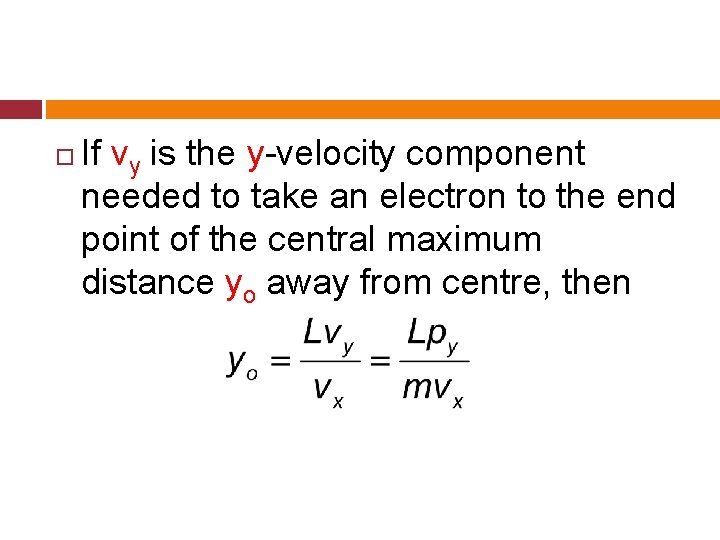
If vy is the y-velocity component needed to take an electron to the end point of the central maximum distance yo away from centre, then

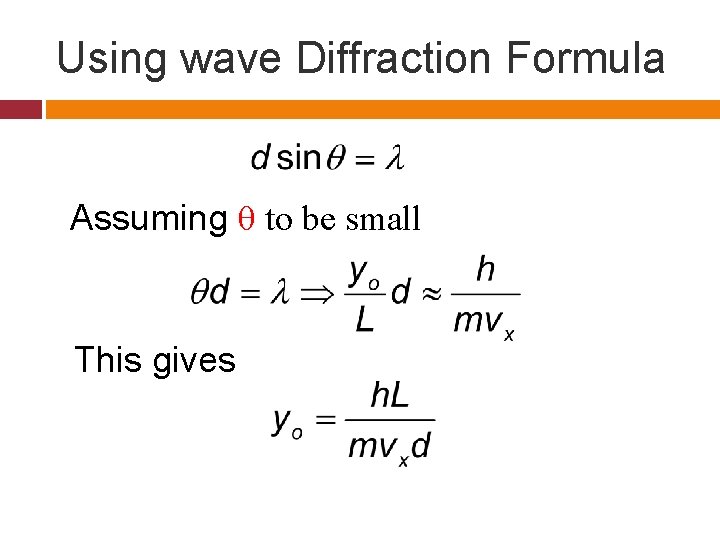
Using wave Diffraction Formula Assuming θ to be small This gives

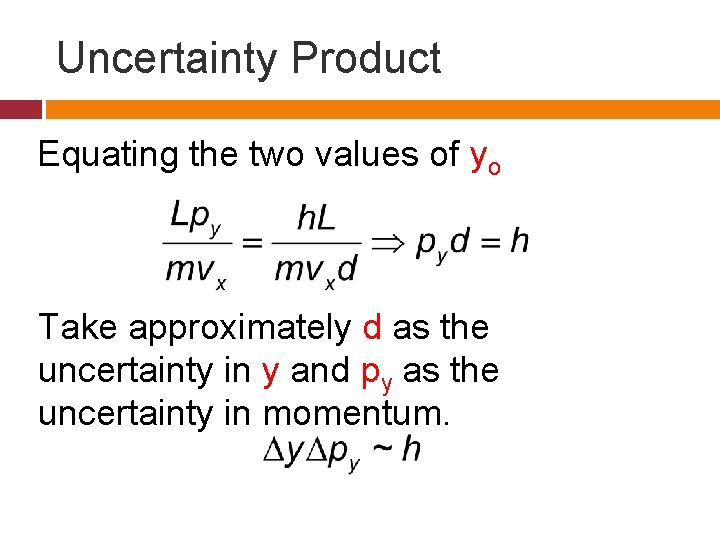
Uncertainty Product Equating the two values of yo Take approximately d as the uncertainty in y and py as the uncertainty in momentum.

Problem In case one is able to ascertain in Young’s double slit experiment that electron is able to pass only through one of the two slits, what should be the maximum allowed uncertainty in y coordinate? Show that this uncertainty will produce an uncertainty in the y component of momentum, that would be enough to destroy the interference

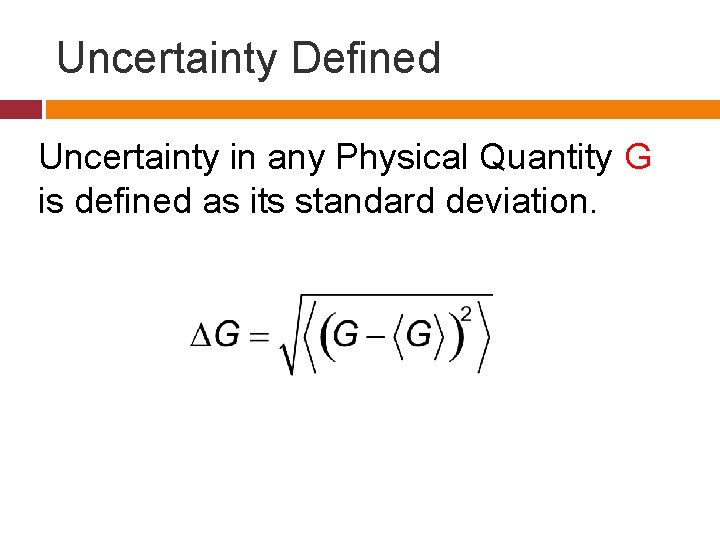
Uncertainty Defined Uncertainty in any Physical Quantity G is defined as its standard deviation.

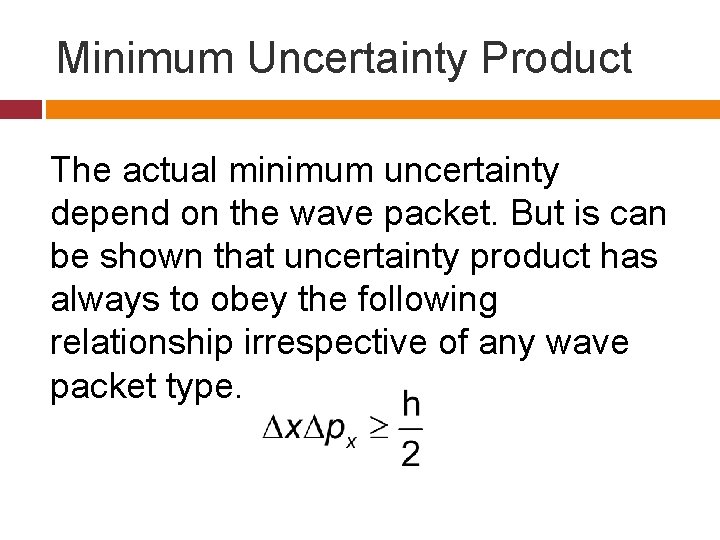
Minimum Uncertainty Product The actual minimum uncertainty depend on the wave packet. But is can be shown that uncertainty product has always to obey the following relationship irrespective of any wave packet type.

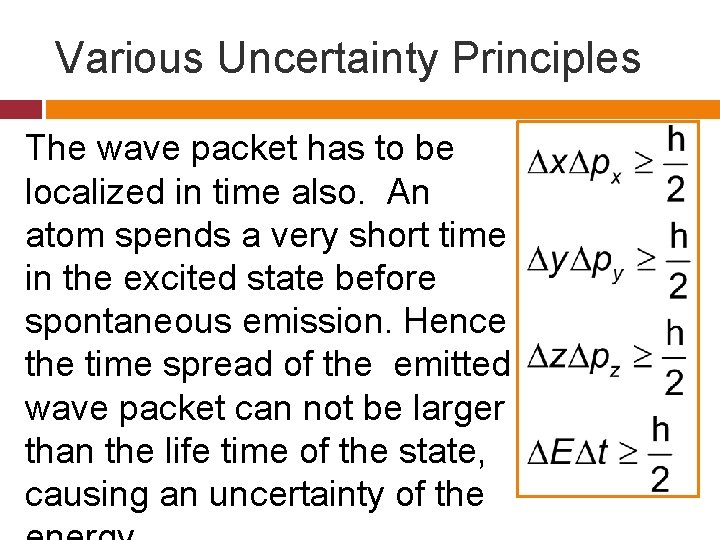
Various Uncertainty Principles The wave packet has to be localized in time also. An atom spends a very short time in the excited state before spontaneous emission. Hence the time spread of the emitted wave packet can not be larger than the life time of the state, causing an uncertainty of the

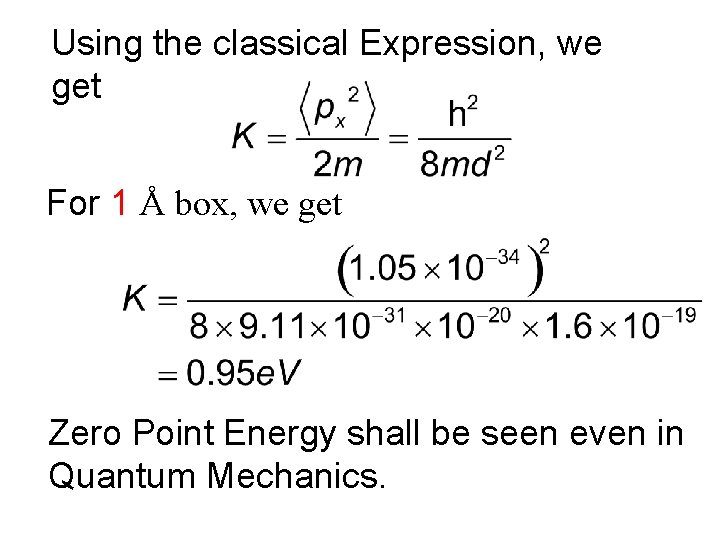
Example: Electron in a Box An electron is confined to a one dimensional ‘box’ of length d. What would be the minimum energy in e. V that the particle should have, if d= 1 Å and 1 Fermi (10 -15 m)

Confining implies This gives Also we expect

Using the classical Expression, we get For 1 Å box, we get Zero Point Energy shall be seen even in Quantum Mechanics.

For 1 fm box, we have to use relativistic expression This is used as an argument that electrons can not be confined in the nucleus.

Example: Harmonic Oscillator. Estimate the minimum possible energy consistent with uncertainty principle (i. e. ground state energy) for a particle of mass m bound in a potential . Use x px /2 for uncertainty principle in this problem.

Let us imagine that the particle is confined to oscillate over a distance of the order of x. Classically if x is small the energy of the oscillator is small. Let us now assume that the particle is represented by a wave packet. Its physical dimension is limited to x. Hence like before, the confinement would cause an uncertainty in momentum and would lead to an average kinetic energy given to the particle.
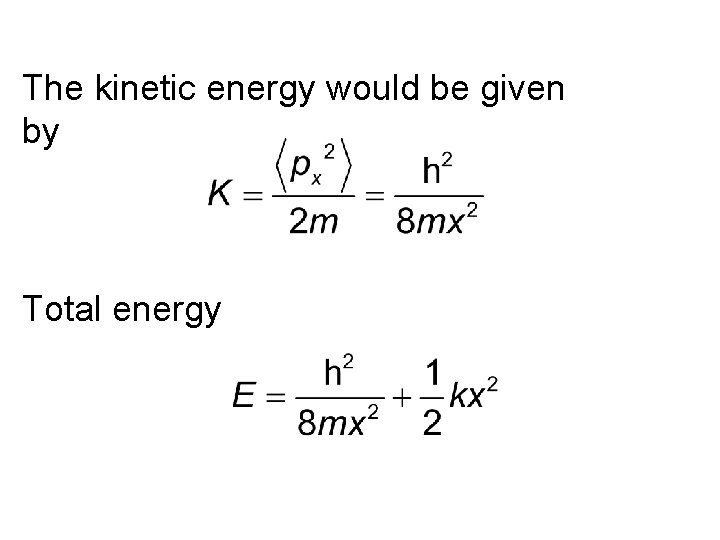
The kinetic energy would be given by Total energy
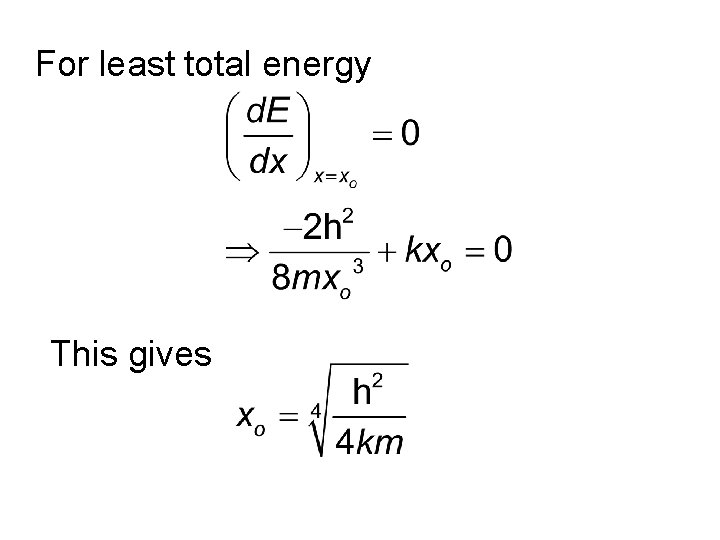
For least total energy This gives

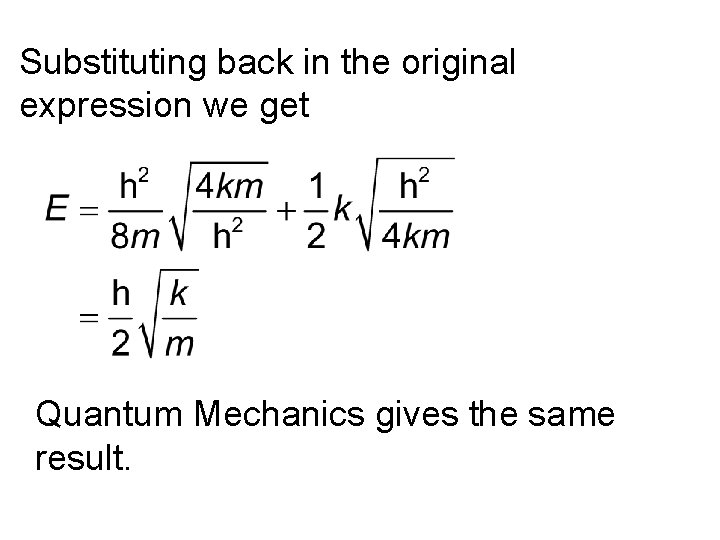
Substituting back in the original expression we get Quantum Mechanics gives the same result.