Waveparticle duality Physics 123 9182020 Lecture XII 1

- Slides: 15

Wave-particle duality Physics 123 9/18/2020 Lecture XII 1

Concepts • • De Broigle waves Energy levels Quantum numbers Emission and absorption spectra 9/18/2020 Lecture XII 2

Wave – Particle duality • If light exhibits both wave and particle properties then particles (e. g. electrons) must also exhibit wave properties – e. g. interference. • Matter (de Broglie) waves l=h/p p=mv 9/18/2020 Lecture XII 3

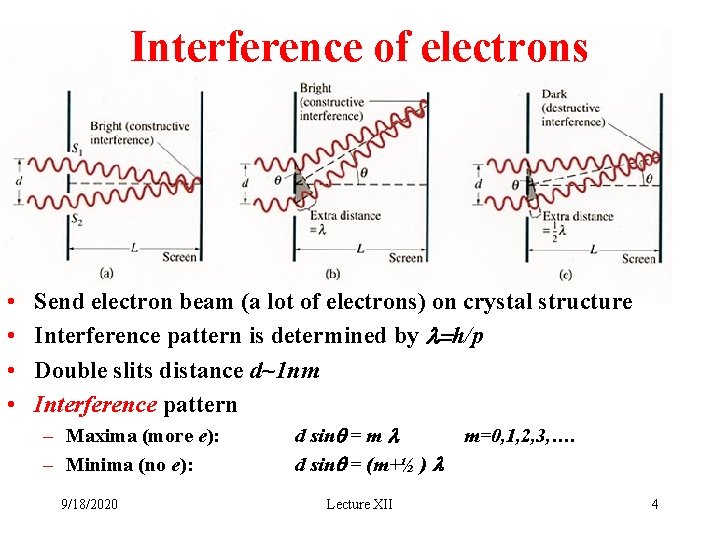
Interference of electrons • • Send electron beam (a lot of electrons) on crystal structure Interference pattern is determined by l=h/p Double slits distance d~1 nm Interference pattern – Maxima (more e): – Minima (no e): 9/18/2020 d sinq = m l d sinq = (m+½ ) l Lecture XII m=0, 1, 2, 3, …. 4

Matter waves • Particle position in space cannot be predicted with infinite precision • Heisenberg uncertainty principle • (Wave function Y of matter wave)2 d. V=probability to find particle in volume d. V. • But while probability is a real number, wave function is a complex number. It has a phase. • When two matter waves meet we add wave functions, not probabilities! Interference can be observed (phase is important 9/18/2020 Lecture XII 5

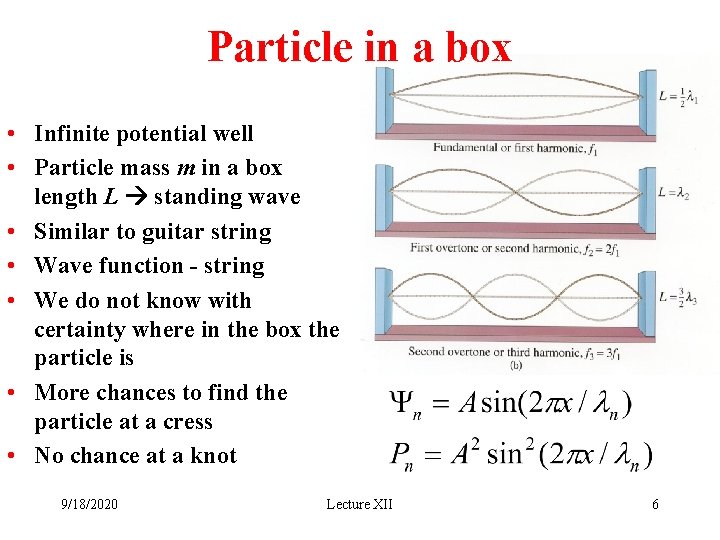
Particle in a box • Infinite potential well • Particle mass m in a box length L standing wave • Similar to guitar string • Wave function - string • We do not know with certainty where in the box the particle is • More chances to find the particle at a cress • No chance at a knot 9/18/2020 Lecture XII 6

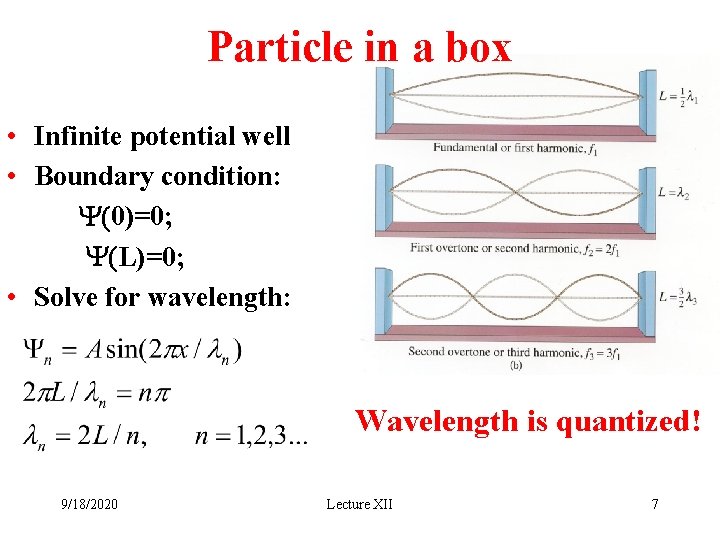
Particle in a box • Infinite potential well • Boundary condition: Y(0)=0; Y(L)=0; • Solve for wavelength: Wavelength is quantized! 9/18/2020 Lecture XII 7

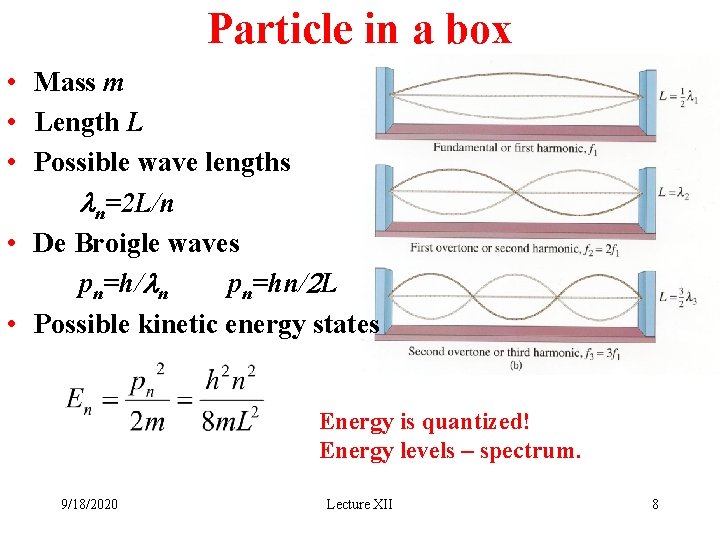
Particle in a box • Mass m • Length L • Possible wave lengths ln=2 L/n • De Broigle waves pn=h/ln pn=hn/2 L • Possible kinetic energy states Energy is quantized! Energy levels – spectrum. 9/18/2020 Lecture XII 8

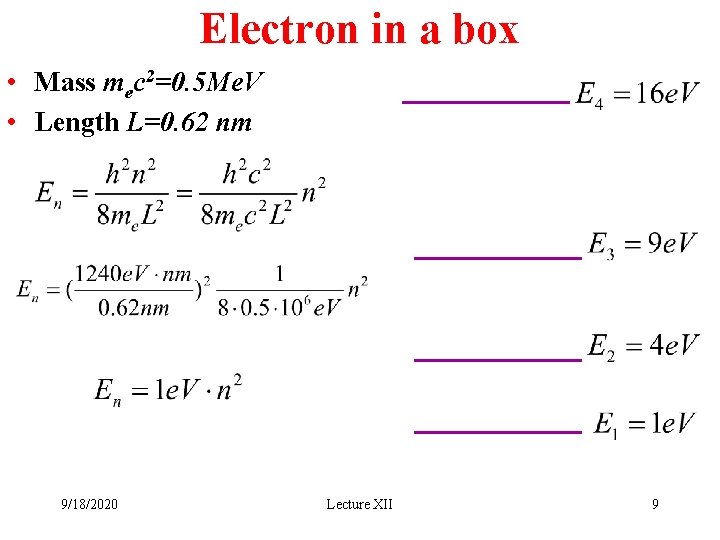
Electron in a box • Mass mec 2=0. 5 Me. V • Length L=0. 62 nm 9/18/2020 Lecture XII 9

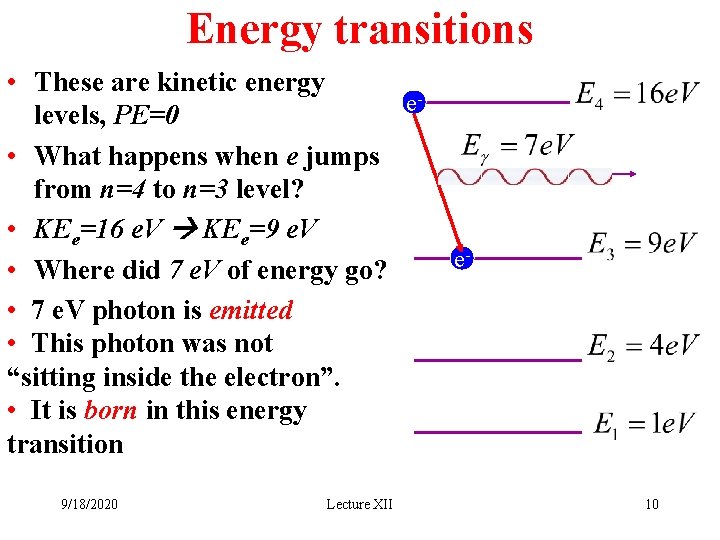
Energy transitions • These are kinetic energy elevels, PE=0 • What happens when e jumps from n=4 to n=3 level? • KEe=16 e. V KEe=9 e. V • Where did 7 e. V of energy go? • 7 e. V photon is emitted • This photon was not “sitting inside the electron”. • It is born in this energy transition 9/18/2020 Lecture XII e- 10

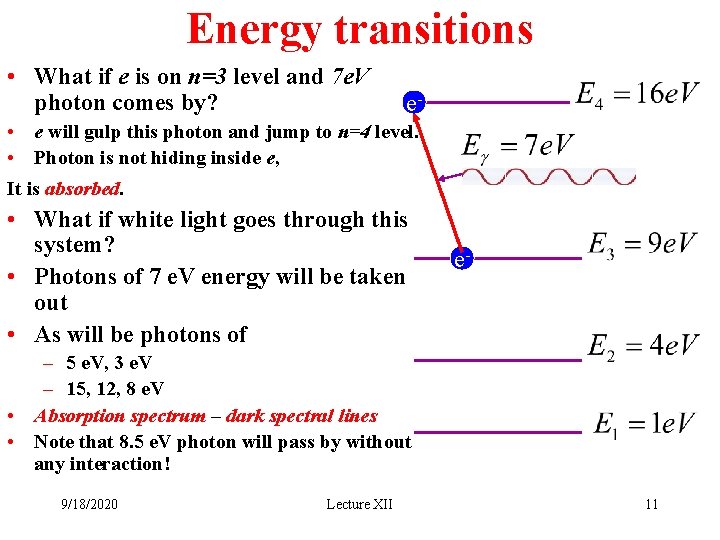
Energy transitions • What if e is on n=3 level and 7 e. V photon comes by? e- • e will gulp this photon and jump to n=4 level. • Photon is not hiding inside e, It is absorbed. • What if white light goes through this system? • Photons of 7 e. V energy will be taken out • As will be photons of e- – 5 e. V, 3 e. V – 15, 12, 8 e. V • Absorption spectrum – dark spectral lines • Note that 8. 5 e. V photon will pass by without any interaction! 9/18/2020 Lecture XII 11

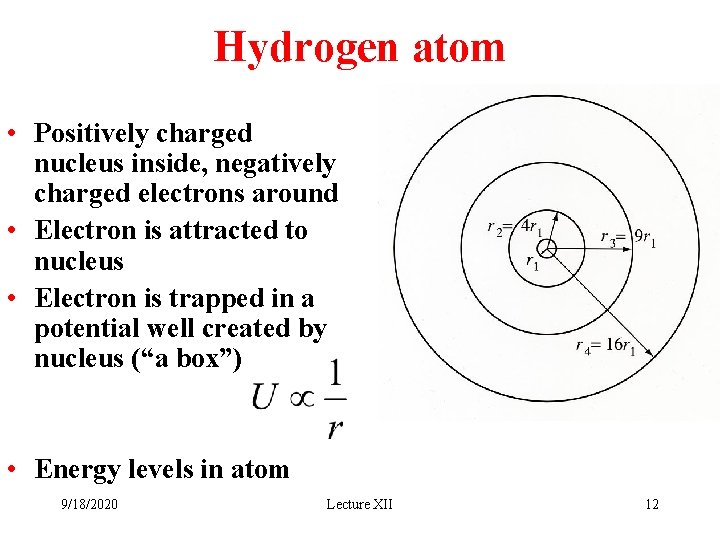
Hydrogen atom • Positively charged nucleus inside, negatively charged electrons around • Electron is attracted to nucleus • Electron is trapped in a potential well created by nucleus (“a box”) • Energy levels in atom 9/18/2020 Lecture XII 12

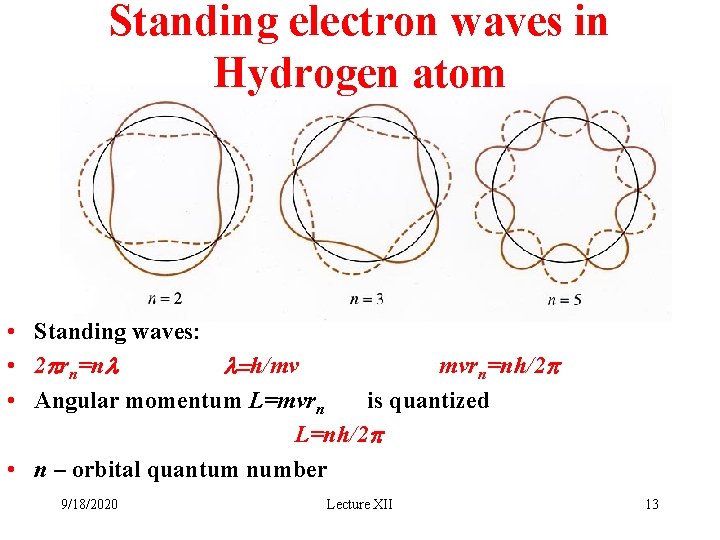
Standing electron waves in Hydrogen atom • Standing waves: • 2 prn=nl l=h/mv mvrn=nh/2 p • Angular momentum L=mvrn is quantized L=nh/2 p • n – orbital quantum number 9/18/2020 Lecture XII 13

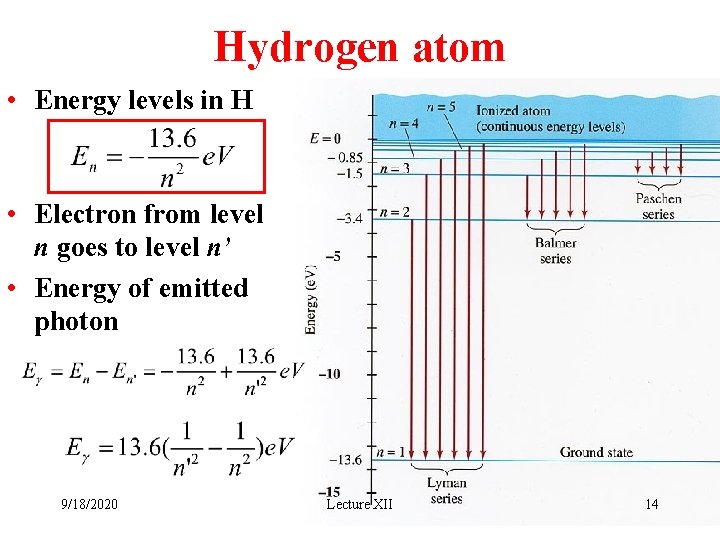
Hydrogen atom • Energy levels in H • Electron from level n goes to level n’ • Energy of emitted photon 9/18/2020 Lecture XII 14

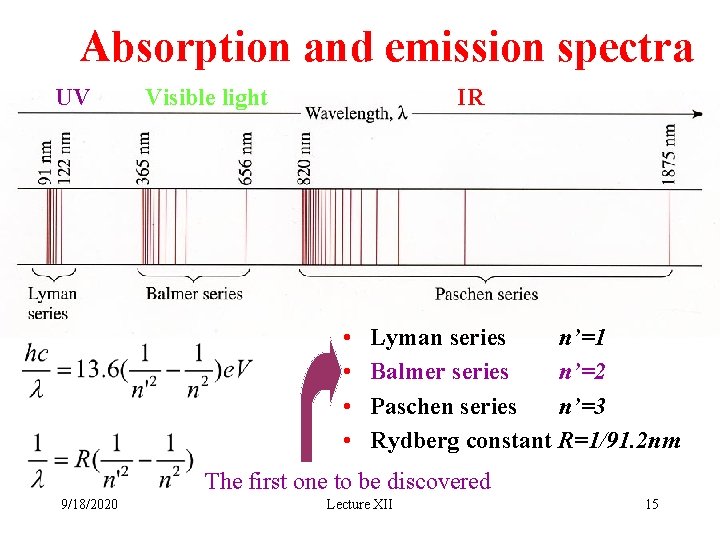
Absorption and emission spectra UV Visible light IR • • Lyman series n’=1 Balmer series n’=2 Paschen series n’=3 Rydberg constant R=1/91. 2 nm The first one to be discovered 9/18/2020 Lecture XII 15