PHY 102 Lecture 13 13 1 WaveParticle Duality




































- Slides: 61

PHY 102: Lecture 13 • 13. 1 Wave-Particle Duality • 13. 2 Blackbody Radiation Planck’s Constant • 13. 3 Photons and Photoelectric Effect • 13. 4 Photon Momentum Compton Effect • 13. 5 de Broglie Wavelength Wave Nature of Matter • 13. 6 Heisenberg Uncertainty Principle

PHY 102: Lecture 12 Particles and Waves 12. 1 Wave-Particle Duality

Wave Interference • Ability to exhibit interference effects is an essential characteristic of waves • An example is Young’s experiment • Light passes through two closely spaced slits and produces a pattern of bright and dark fringes on a screen • The fringe pattern is a direct indication that interference is occurring between light waves coming from each slit

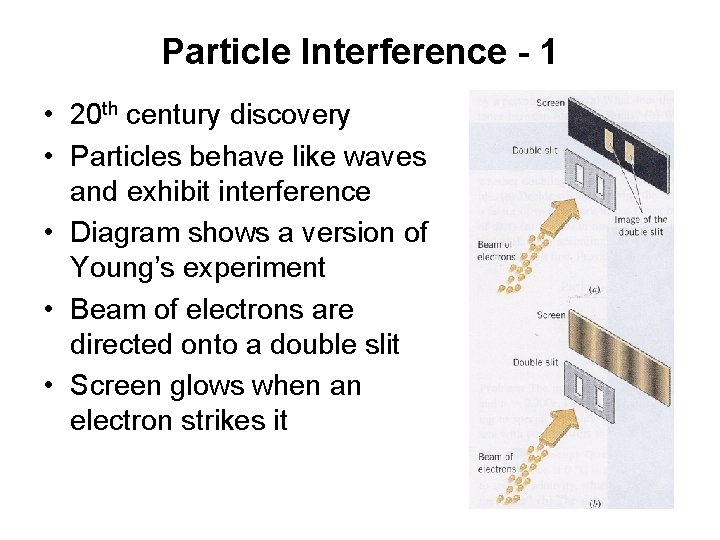
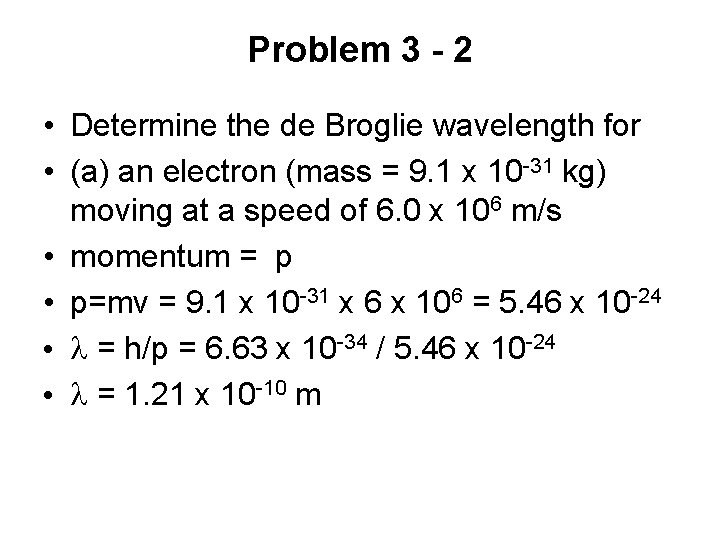
Particle Interference - 1 • 20 th century discovery • Particles behave like waves and exhibit interference • Diagram shows a version of Young’s experiment • Beam of electrons are directed onto a double slit • Screen glows when an electron strikes it

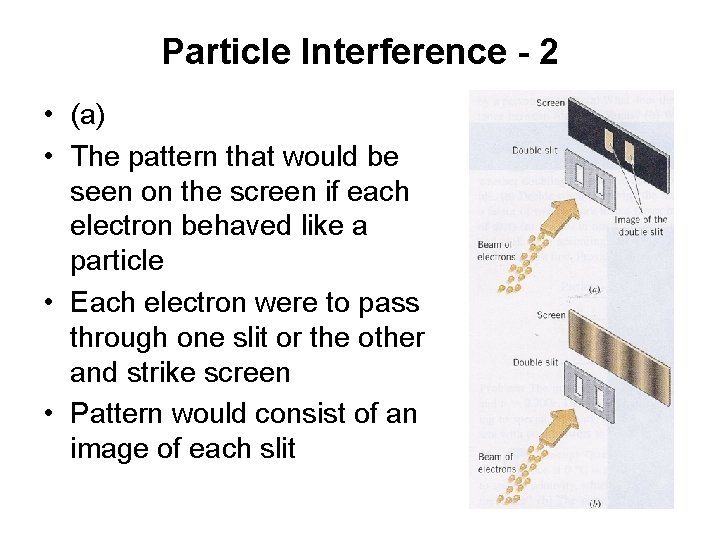
Particle Interference - 2 • (a) • The pattern that would be seen on the screen if each electron behaved like a particle • Each electron were to pass through one slit or the other and strike screen • Pattern would consist of an image of each slit

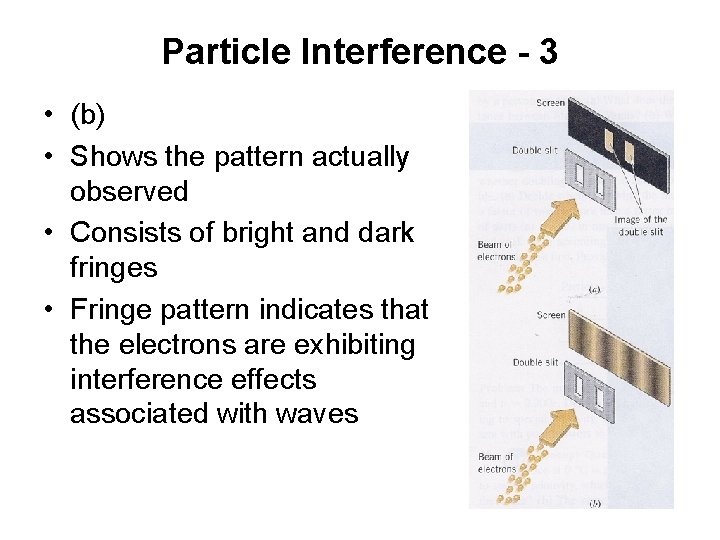
Particle Interference - 3 • (b) • Shows the pattern actually observed • Consists of bright and dark fringes • Fringe pattern indicates that the electrons are exhibiting interference effects associated with waves

Wave-Particle Duality • The concept of an electron as a tiny discrete particle of matter does not account for the fact that the electron can behave as a wave in some circumstances • The electron exhibits a dual nature, with both particle-like characteristics and wavelike characteristics • Waves can exhibit particle-like characteristics, and particles can exhibit wave-like characteristics

PHY 102: Lecture 12 Particles and Waves 12. 2 Blackbody Radiation; Planck’s Constant

Thermal Radiation - 1 • All bodies continuously radiate electromagnetic waves (blackbody radiation) • We see the glow of very hot objects because they emit electromagnetic waves in the visible region of the spectrum • At low temperatures objects emit visible light waves only weakly and do not appear to be glowing

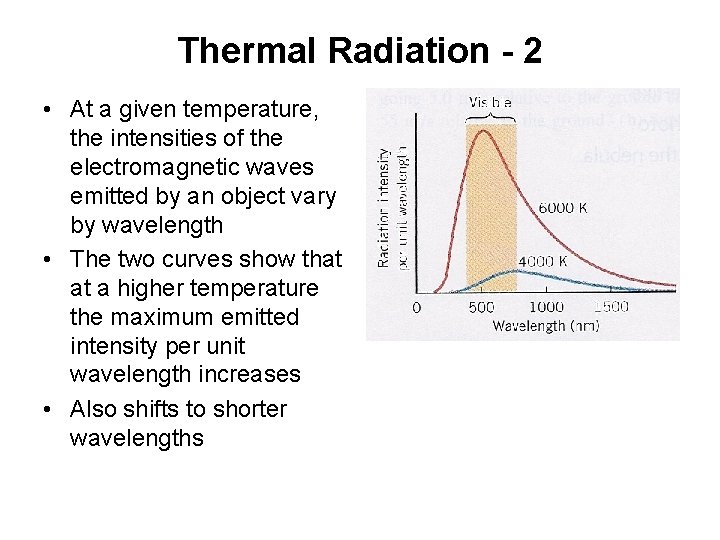
Thermal Radiation - 2 • At a given temperature, the intensities of the electromagnetic waves emitted by an object vary by wavelength • The two curves show that at a higher temperature the maximum emitted intensity per unit wavelength increases • Also shifts to shorter wavelengths

Max Planck (1858 – 1947) • • 1900 German Explained thermal radiation curves Took first step toward understanding of the wave-particle duality

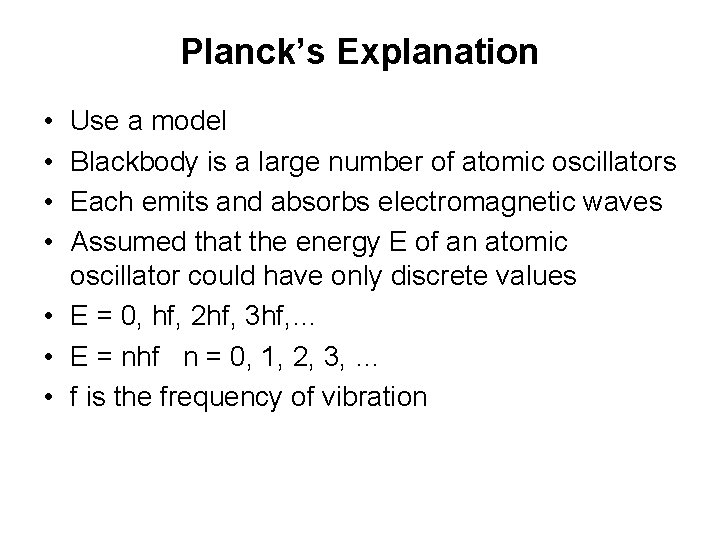
Planck’s Explanation • • Use a model Blackbody is a large number of atomic oscillators Each emits and absorbs electromagnetic waves Assumed that the energy E of an atomic oscillator could have only discrete values • E = 0, hf, 2 hf, 3 hf, … • E = nhf n = 0, 1, 2, 3, … • f is the frequency of vibration

Planck’s Constant • h is Planck’s constant • h = 6. 62606876 x 10 -34 Js

Quantized Energy • Radical feature of Planck’s assumption • Energy of an atomic oscillator could have only discrete values • hf, 2 hf, 3 hf, … • Energies in between these values are forbidden • Whenever the energy of a system can have only certain definite values and nothing in between the energy is quantized

Quantized Electromagnetic Energy • Conservation of energy requires that the energy carried off by the radiated electromagnetic waves must equal the energy lost by the atomic oscillators • For example, an oscillator with an energy of 3 hf emits an electromagnetic wave • Next smallest allowed value for the energy of the oscillator is 2 hf • Energy carried off by the electromagnetic wave would have the value of hf

PHY 102: Lecture 12 Particles and Waves 12. 3 Photons and Photoelectric Effect

Photons • Electromagnetic waves are composed of particle-like entities called photons • Energy = E = hf • Momentum = p = h/l

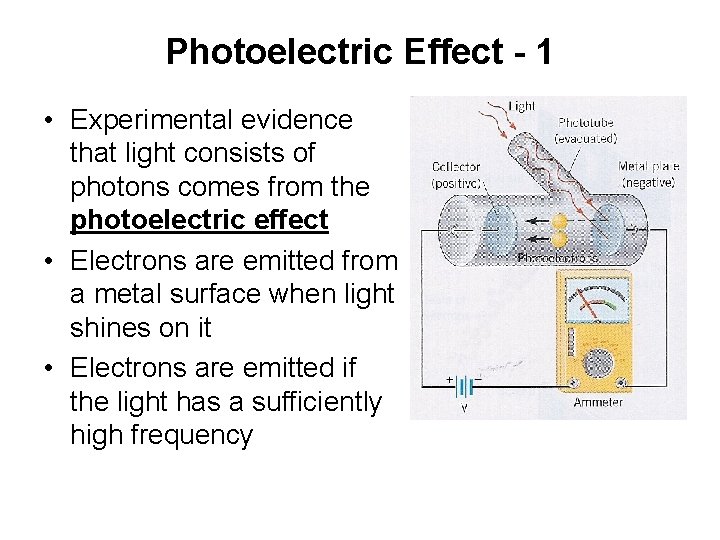
Photoelectric Effect - 1 • Experimental evidence that light consists of photons comes from the photoelectric effect • Electrons are emitted from a metal surface when light shines on it • Electrons are emitted if the light has a sufficiently high frequency

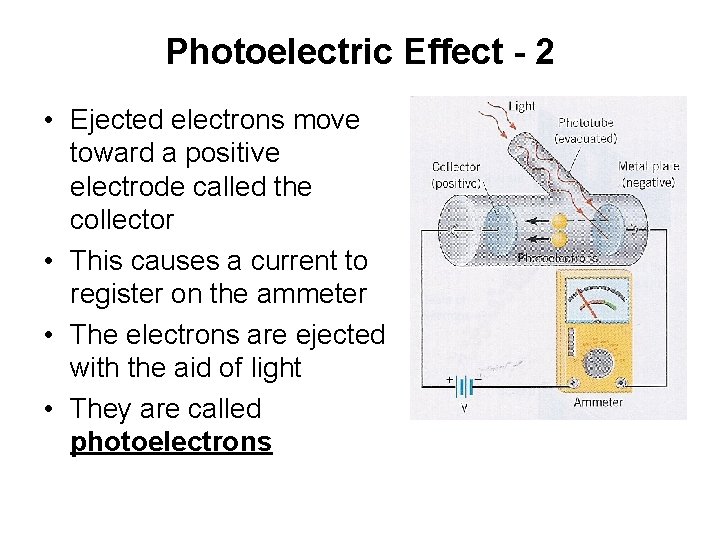
Photoelectric Effect - 2 • Ejected electrons move toward a positive electrode called the collector • This causes a current to register on the ammeter • The electrons are ejected with the aid of light • They are called photoelectrons

Photoelectric Effect - 3 • In 1905 Einstein presented an explanation of the photoelectric • Einstein proposed that light of frequency f could be regarded as a collection of discrete packets of energy (photons) • Each packet containing an amount of energy given by • E = hf • h is Planck’s constant

Photoelectric Effect - 4 • Einstein explained that when light shines on a metal, a photon can give up its energy to an electron in the metal • If the photon has enough energy to do the work of removing the electron from the metal, the electron can be ejected • Work required depends on how strongly the electron is held • For least strongly held electrons, the work has a minimum value W 0, called work function

Photoelectric Effect - 5 • If a photon has energy in excess of the work needed to remove an electron, the excess appears as kinetic energy of the ejected electron

Photoelectric Effect Equation • Photon energy = Max. KE of ejected electron + Min. work to eject electron • • hf = KEmax + W 0 KEmax = hf – W 0 If KEmax = 0 then hf 0 = W 0 is the work function

Problem 1 - 1 • In converting electrical energy into light energy, a sixty-watt incandescent light bulb operates at about 2. 1% efficiency • Assuming that all the light is green (vacuum wavelength = 5. 55 x 10 -7 m) • Determine the number of photons per second given off by the bulb

Problem 1 - 2 • At an efficiency of 2. 1% the light energy emitted per second by a sixty-watt bulb is • (0. 021)(60. 0 J/s) = 1. 3 J/s • The energy of a single photon is • E = hf = hc/l • E = (6. 63 x 10 -34)(3. 00 x 108)/5. 55 x 10 -7 • E = 3. 58 x 10 -19 J • Photons / sec = 1. 3/3. 58 x 10 -19 =3. 6 x 1018

Problem 2 - 1 • Work function for silver surface is W 0 = 4. 73 e. V • Find the minimum frequency that light must have to eject electrons from this surface üW 0 = (4. 73 e. V)(1. 60 x 10 -19 J/1 ev) üW 0 = 3. 58 x 10 -19 J ühf 0 = KEmax +W 0 = 0 + W 0 üf 0 = W 0/h = 7. 57 x 10 -19 J/6. 63 x 10 -34 Js üf 0 = 1. 14 x 1015 Hz ül 0 = 263 nm ultraviolet

Classical vs Modern Physics - 1 • Classical Physics • Kinetic energy of electron independent of frequency • Modern Physics • Only light with a frequency above a minimum value will eject electrons • Regardless of brightness of light

Classical vs Modern Physics - 2 • Classical Physics • Maximum kinetic energy of electron increases as brightness of light increases • Modern Physics • Maximum kinetic energy remains the same as the brightness increases • As brightness increases, the number of electrons ejected increases

Classical vs Modern Physics - 3 • Classical Physics • For a dim beam of light, there should be a long delay before electrons are emitted • Modern Physics • Electrons are emitted immediately

PHY 102: Lecture 12 Particles and Waves 12. 4 Photon Momentum / Compton Effect

Arthur H. Compton (1892 – 1962) • • 1923 American Physicist Used photon model to explain scattering of X-rays by the electrons in graphite • X-rays are high-frequency electromagnetic waves and are composed of photons

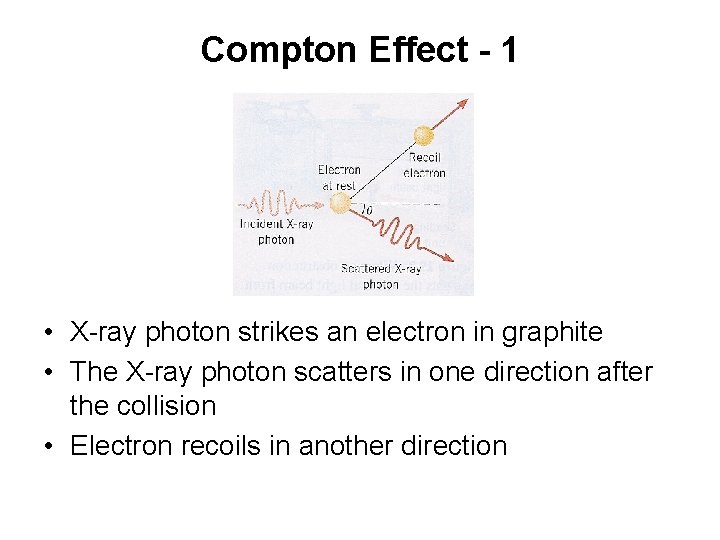
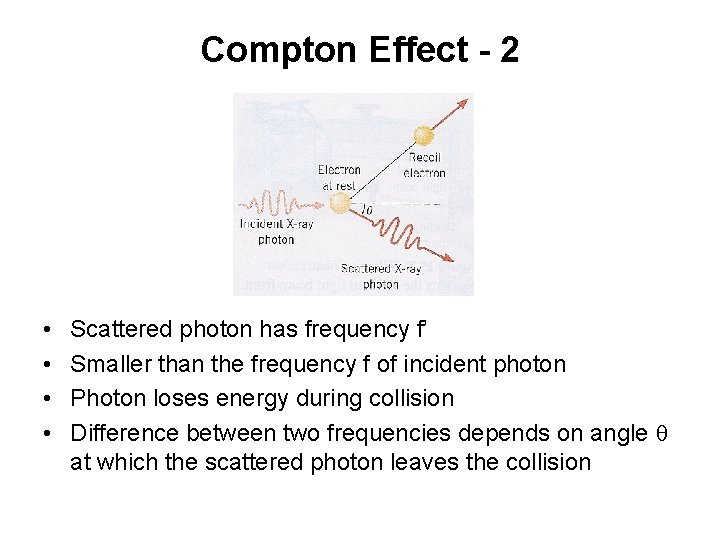
Compton Effect - 1 • X-ray photon strikes an electron in graphite • The X-ray photon scatters in one direction after the collision • Electron recoils in another direction

Compton Effect - 2 • • Scattered photon has frequency f’ Smaller than the frequency f of incident photon Photon loses energy during collision Difference between two frequencies depends on angle q at which the scattered photon leaves the collision

Compton Effect Conservation of Energy / Momentum • Energy of incident photon = Energy of scattered photon + Kinetic energy of recoil electron • hf = hf’ + KE • hf’ = hf – KE • Energy and frequency of scattered photon is less than energy and frequency of incident photon • Momentum of incident photon = Momentum of scattered photon + Momentum of recoil electron

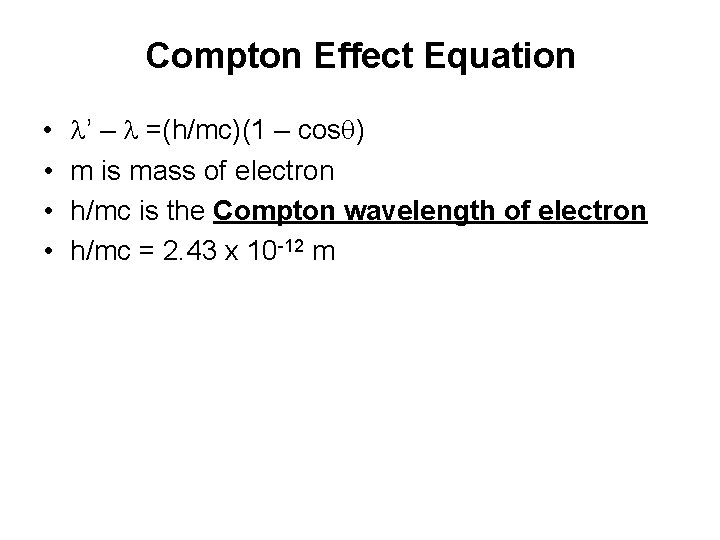
Compton Effect Equation • • l’ – l =(h/mc)(1 – cosq) m is mass of electron h/mc is the Compton wavelength of electron h/mc = 2. 43 x 10 -12 m

PHY 102: Lecture 12 Particles and Waves 12. 5 de Broglie Wavelength / Wave Nature of Light

Louis de Broglie (1892 – 1987) • 1923 • French • Suggested that since light waves could exhibit particle-like behavior, particles of matter should exhibit wave-like behavior • de Broglie wavelength • l = h/p – h is Planck’s constant – p is magnitude of relativistic momentum of the particle

Clinton J. Davisson (1881 – 1958) Lester H. Germer (1896 - 1971 • 1927 • American • Directed a beam of electrons on a crystal of nickel • Observed that the electrons exhibited a diffraction behavior

Neutron Diffraction Pattern

Problem 3 - 1 • Determine the de Broglie wavelength for • (a) an electron (mass = 9. 1 x 10 -31 kg) moving at a speed of 6. 0 x 106 m/s • (b) a baseball (mass = 0. 15 kg) moving at a speed of 13 m/s

Problem 3 - 2 • Determine the de Broglie wavelength for • (a) an electron (mass = 9. 1 x 10 -31 kg) moving at a speed of 6. 0 x 106 m/s • momentum = p • p=mv = 9. 1 x 10 -31 x 6 x 106 = 5. 46 x 10 -24 • l = h/p = 6. 63 x 10 -34 / 5. 46 x 10 -24 • l = 1. 21 x 10 -10 m

Problem 3 - 3 • Determine the de Broglie wavelength for • (b) a baseball (mass = 0. 15 kg) moving at a speed of 13 m/s • momentum = p • p = mv = 0. 15 x 13 = 1. 95 • l = h/p = 6. 63 x 10 -34 / 1. 95 • l = 3. 4 x 10 -34 m

What Kind of Wave is de Broglie Wave? -1 • (a) Shows the fringe pattern on the screen when electrons are used in a version of Young’s double-slit experiment • Bright fringes occur in places where particle waves coming from each slit interfere constructively • Dark fringes occur in places where the waves interfere destructively

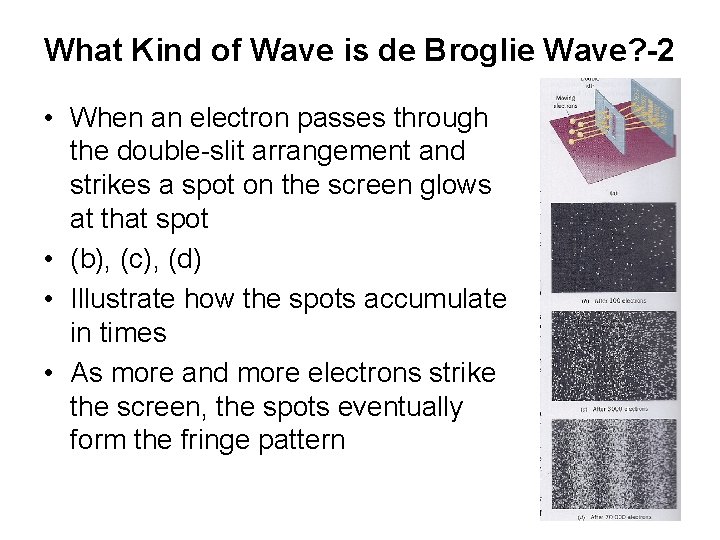
What Kind of Wave is de Broglie Wave? -2 • When an electron passes through the double-slit arrangement and strikes a spot on the screen glows at that spot • (b), (c), (d) • Illustrate how the spots accumulate in times • As more and more electrons strike the screen, the spots eventually form the fringe pattern

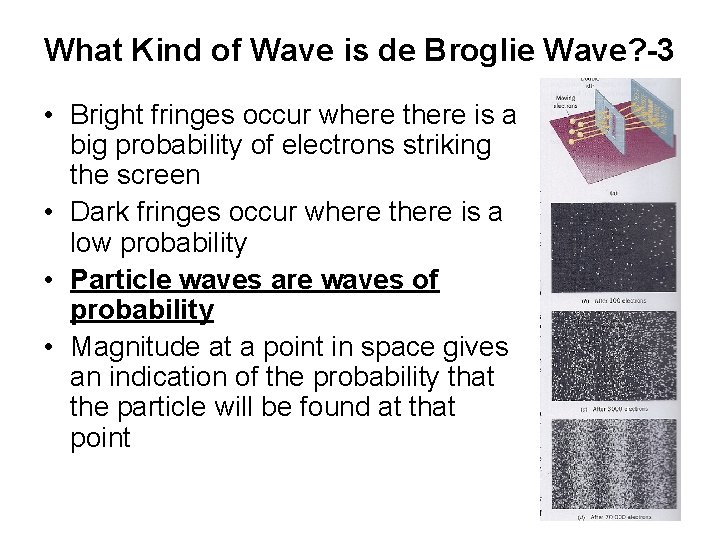
What Kind of Wave is de Broglie Wave? -3 • Bright fringes occur where there is a big probability of electrons striking the screen • Dark fringes occur where there is a low probability • Particle waves are waves of probability • Magnitude at a point in space gives an indication of the probability that the particle will be found at that point

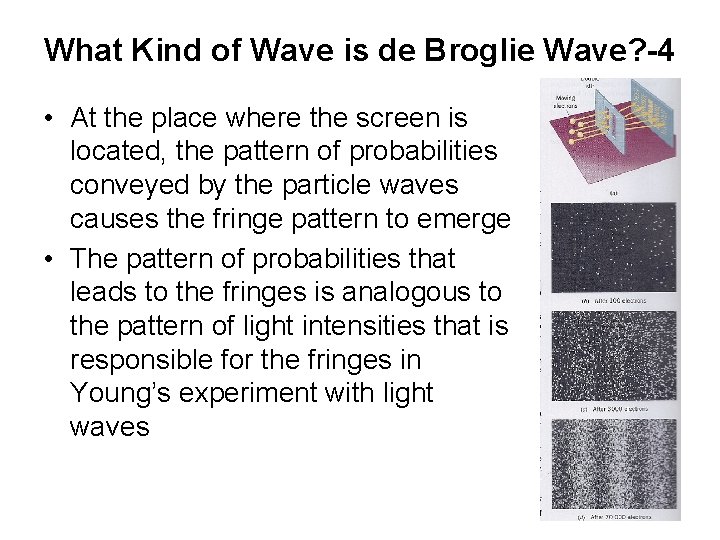
What Kind of Wave is de Broglie Wave? -4 • At the place where the screen is located, the pattern of probabilities conveyed by the particle waves causes the fringe pattern to emerge • The pattern of probabilities that leads to the fringes is analogous to the pattern of light intensities that is responsible for the fringes in Young’s experiment with light waves

Wavefunction • In the case of particle waves, the probability is proportional to the square of the magnitude Y • Y is the wave function of the particle • In 1925, Erwin Schrodinger and Werner Heisenberg developed theoretical frameworks for determining the wave function • Established a new branch of physics called quantum mechanics

PHY 102: Lecture 12 Particles and Waves 12. 6 Heisenberg Uncertainty Principle

Uncertainty - 1 • Bright fringes indicate the places where there is a high probability of an electron striking the screen • There a number of bright fringes • There is more than one place where each electron has some probability of hitting • It is not possible to specify in advance exactly where on screen an individual electron will hit • All we can do is speak of probability that electron may end up in a number of different places

Uncertainty - 2 • No longer is it possible to say, as Newton’s laws would suggest, that a single electron, fired through the double slit, will travel directly forward in a straight line and strike the screen • This simple model does not apply when a particle as small as an electron passes through a pair of closely spaced narrow slits • Wave nature of particles is important in such circumstances, we lose the ability to predict with 100% certainty the path that a single particle will follow

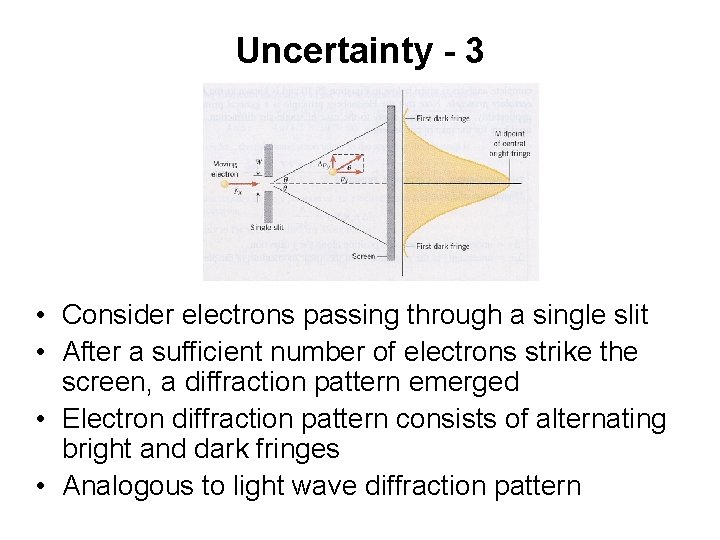
Uncertainty - 3 • Consider electrons passing through a single slit • After a sufficient number of electrons strike the screen, a diffraction pattern emerged • Electron diffraction pattern consists of alternating bright and dark fringes • Analogous to light wave diffraction pattern

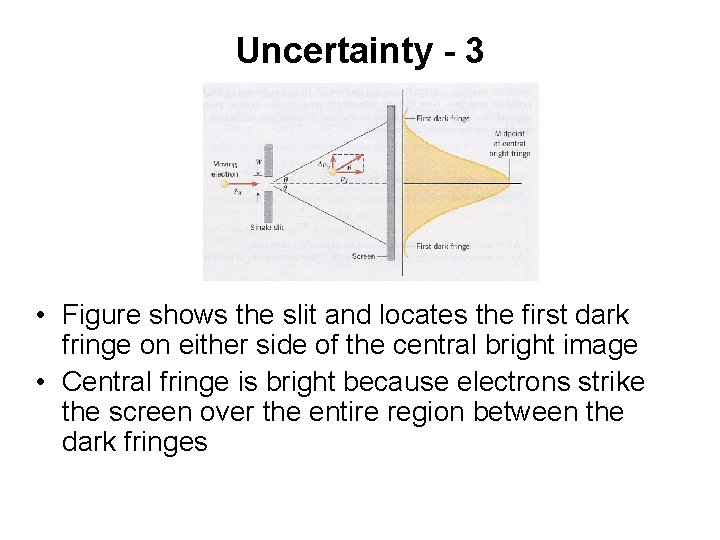
Uncertainty - 3 • Figure shows the slit and locates the first dark fringe on either side of the central bright image • Central fringe is bright because electrons strike the screen over the entire region between the dark fringes

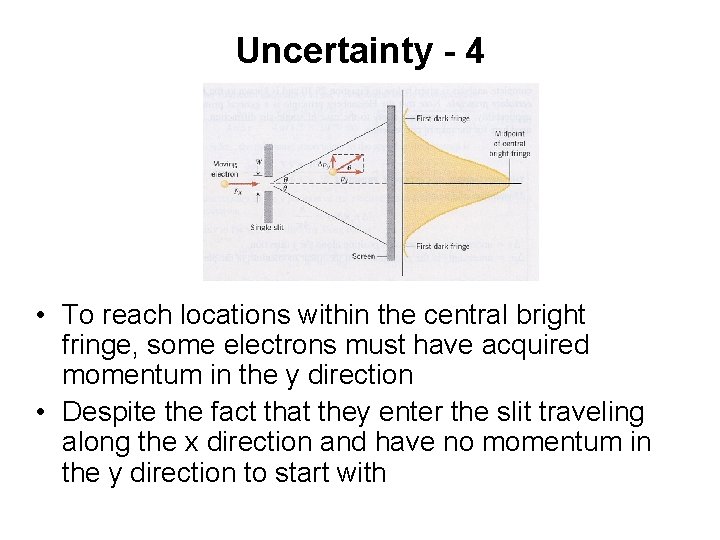
Uncertainty - 4 • To reach locations within the central bright fringe, some electrons must have acquired momentum in the y direction • Despite the fact that they enter the slit traveling along the x direction and have no momentum in the y direction to start with

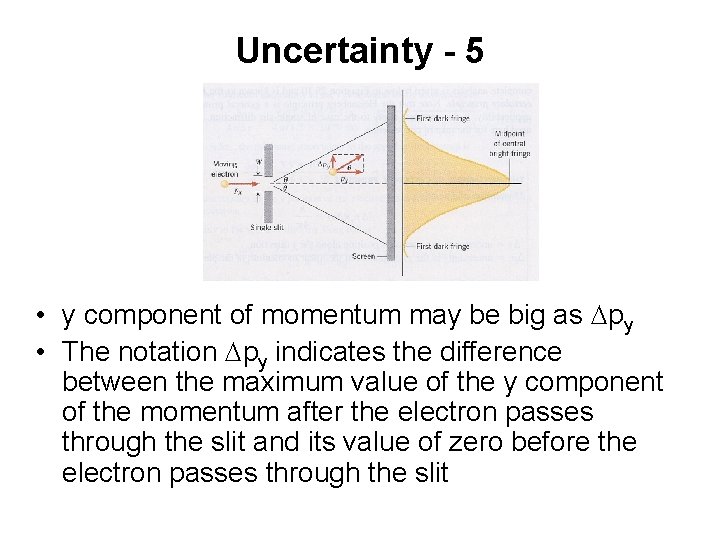
Uncertainty - 5 • y component of momentum may be big as Dpy • The notation Dpy indicates the difference between the maximum value of the y component of the momentum after the electron passes through the slit and its value of zero before the electron passes through the slit

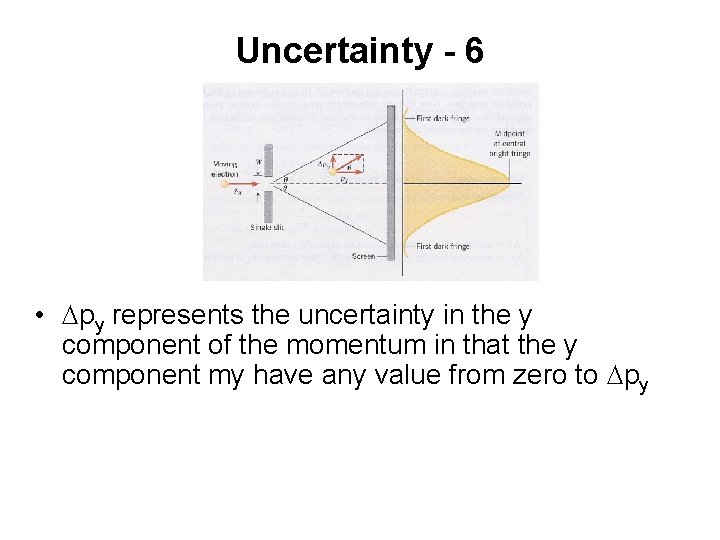
Uncertainty - 6 • Dpy represents the uncertainty in the y component of the momentum in that the y component my have any value from zero to Dpy

Uncertainty - 7 • Assume that light diffraction equation also applies to particle whose wavelength is l • sinq = l/W locate first dark fringe • If q is small then sinq tanq • tanq = Dpy/px

Uncertainty - 8 • Dpy / px = Dpy / (h/l) = l/W • Dpy / px = h / W • Heisenberg suggested that the uncertainty Dpy is related to the uncertainty in the y position of the electron as the electron passes through the slit

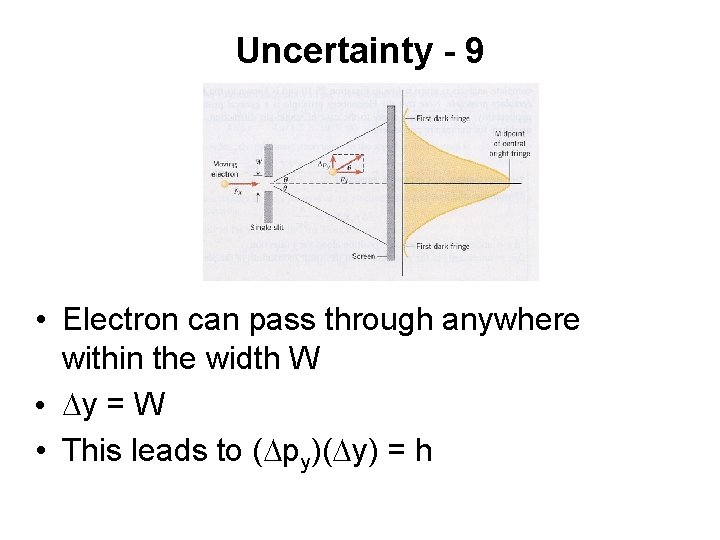
Uncertainty - 9 • Electron can pass through anywhere within the width W • Dy = W • This leads to (Dpy)(Dy) = h

Heisenberg Uncertainty Principle • Momentum and position • (Dpy)(Dy) >= h/4 p • Dy = uncertainty in a particle’s position along the x direction • Dpy = uncertainty in the y component of the linear momentum of the particle • Energy and Time • (DE)(Dt) >= h/4 p • DE = uncertainty in the energy of a particle when the particle is in a certain state • Dt = time interval during which the particle is in the state

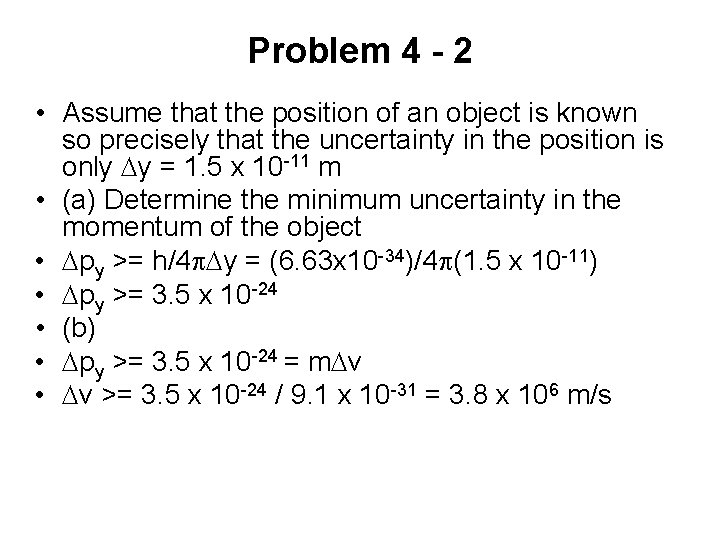
Problem 4 - 1 • Assume that the position of an object is known so precisely that the uncertainty in the position is only Dy = 1. 5 x 10 -11 m • (a) Determine the minimum uncertainty in the momentum of the object • (b) Find the corresponding minimum uncertainty in the speed of the object in the case when the object is an electron (mass = 9. 1 x 10 -31 kg)

Problem 4 - 2 • Assume that the position of an object is known so precisely that the uncertainty in the position is only Dy = 1. 5 x 10 -11 m • (a) Determine the minimum uncertainty in the momentum of the object • Dpy >= h/4 p. Dy = (6. 63 x 10 -34)/4 p(1. 5 x 10 -11) • Dpy >= 3. 5 x 10 -24 • (b) • Dpy >= 3. 5 x 10 -24 = m. Dv • Dv >= 3. 5 x 10 -24 / 9. 1 x 10 -31 = 3. 8 x 106 m/s