Reactions in Aqueous Solution Chapter 4 Part 3












- Slides: 21

Reactions in Aqueous Solution Chapter 4 – Part 3

Acid Base Reactions Proton Transfer Reactions AKA - Neutralization

Acids: n n Substances that increase the concentration of H+ when dissolved in water (Arrhenius). Proton donors (Brønsted–Lowry).

Acids There are only seven strong acids:

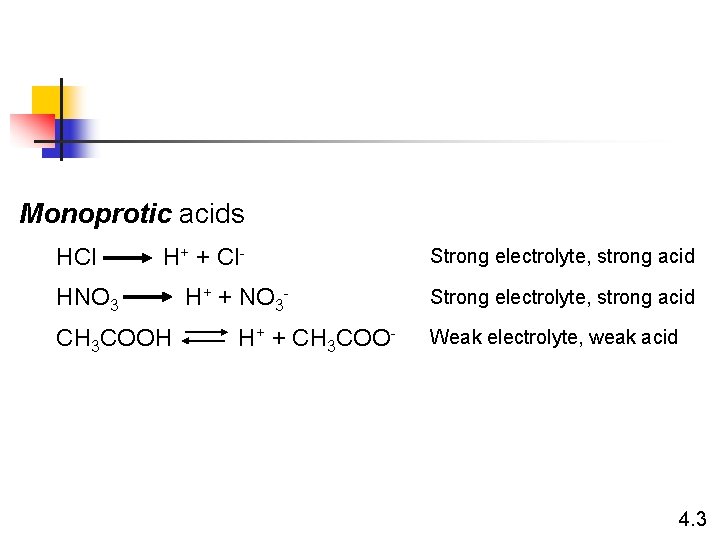
Monoprotic acids HCl H+ + Cl- HNO 3 CH 3 COOH H+ + NO 3 H+ + CH 3 COO- Strong electrolyte, strong acid Weak electrolyte, weak acid 4. 3

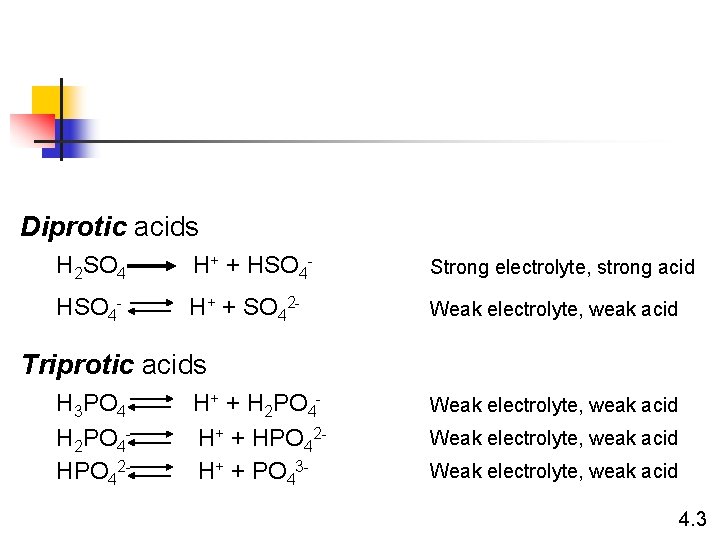
Diprotic acids H 2 SO 4 H+ + HSO 4 - Strong electrolyte, strong acid HSO 4 - H+ + SO 42 - Weak electrolyte, weak acid Triprotic acids H 3 PO 4 H 2 PO 4 HPO 42 - H+ + H 2 PO 4 H+ + HPO 42 H+ + PO 43 - Weak electrolyte, weak acid 4. 3

Bases: n n Substances that increase the concentration of OH− when dissolved in water (Arrhenius). Proton acceptors (Brønsted–Lowry).

Bases The strong bases are the soluble salts of hydroxide ion:

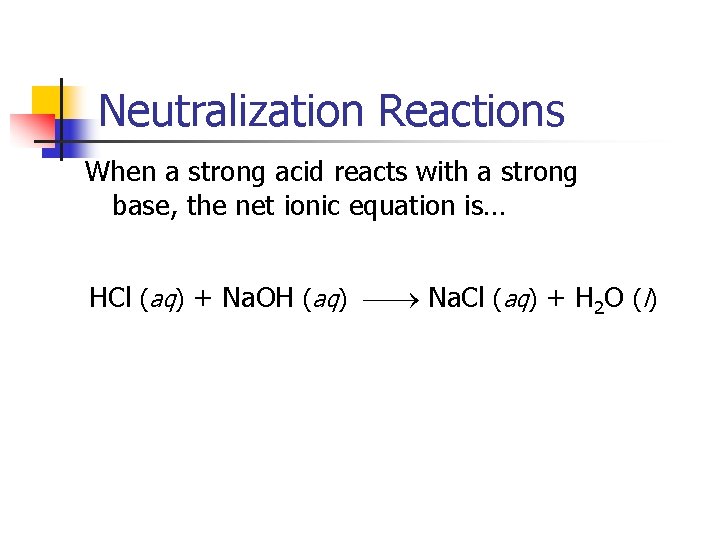
Neutralization Reactions Generally, when solutions of an acid and a base are combined, the products are a salt and water. HCl (aq) + Na. OH (aq) Na. Cl (aq) + H 2 O (l) Salts are less reactive than acids and bases. Why do they do that?

Neutralization Reactions When a strong acid reacts with a strong base, the net ionic equation is… HCl (aq) + Na. OH (aq) Na. Cl (aq) + H 2 O (l)

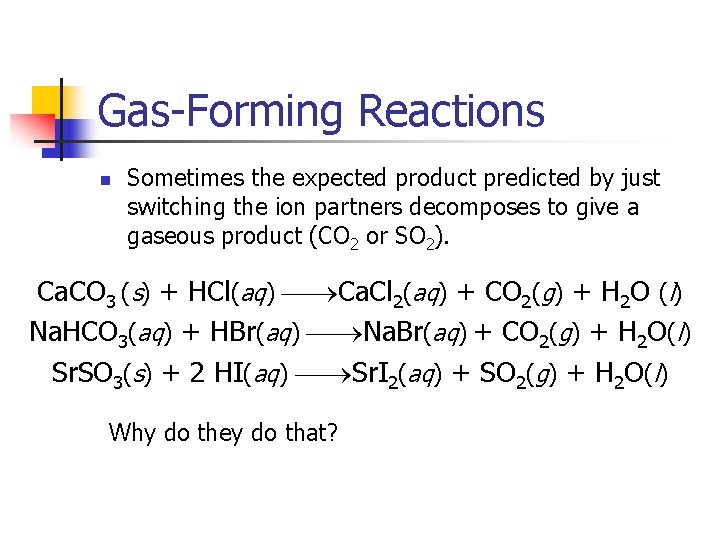
Gas-Forming Reactions n Sometimes the expected product predicted by just switching the ion partners decomposes to give a gaseous product (CO 2 or SO 2). Ca. CO 3 (s) + HCl(aq) Ca. Cl 2(aq) + CO 2(g) + H 2 O (l) Na. HCO 3(aq) + HBr(aq) Na. Br(aq) + CO 2(g) + H 2 O(l) Sr. SO 3(s) + 2 HI(aq) Sr. I 2(aq) + SO 2(g) + H 2 O(l) Why do they do that?

Gas-Forming Reactions n n This reaction gives the predicted product, but you had better carry it out in the hood, or you will be very unpopular! Just as in the previous examples, a gas is formed as a product of this reaction: Na 2 S (aq) + H 2 SO 4 (aq) Na 2 SO 4 (aq) + H 2 S (g)

Solution Stoichiometry Count with Volume!

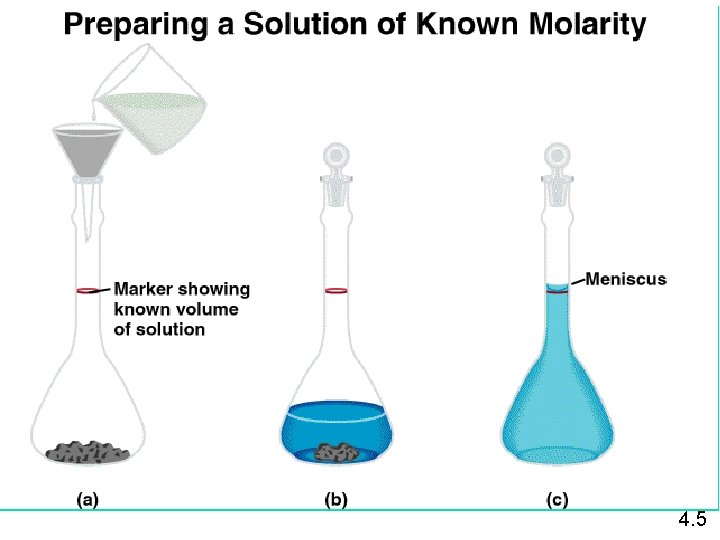
Molarity n n Two solutions can contain the same compounds but be quite different because the proportions of those compounds are different. Molarity is one way to measure the concentration of a solution. moles of solute Molarity (M) = volume of solution in liters

4. 5

Making Solutions M = molarity = moles of solute liters of solution What mass of KI is required to make 500. m. L of a 2. 80 M KI solution? 4. 5

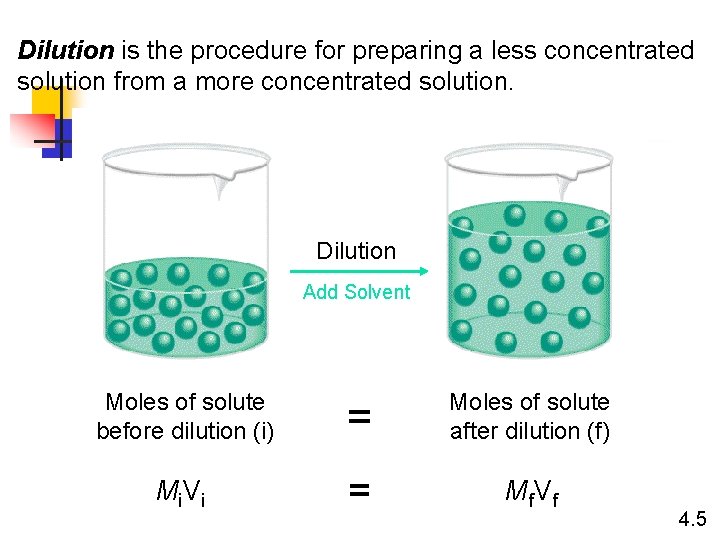
Dilution is the procedure for preparing a less concentrated solution from a more concentrated solution. Dilution Add Solvent Moles of solute before dilution (i) = Moles of solute after dilution (f) Mi V i = Mf V f 4. 5

How would you prepare 60. 0 m. L of 0. 200 M HNO 3 from a stock solution of 4. 00 M HNO 3? 4. 5

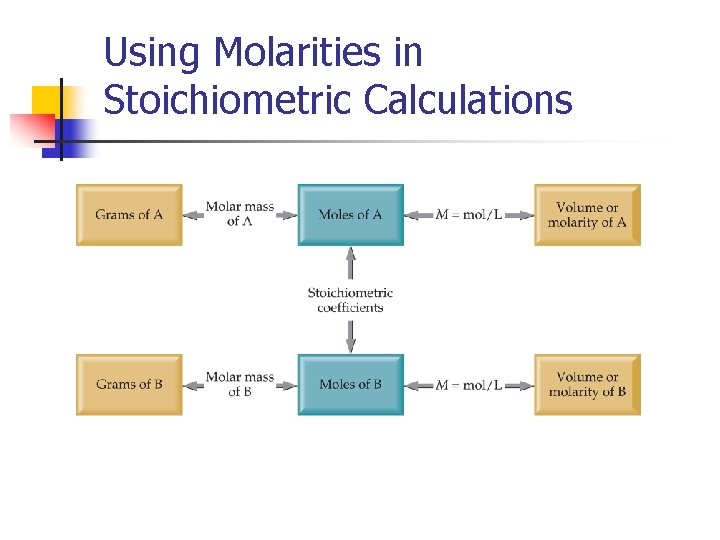
Using Molarities in Stoichiometric Calculations

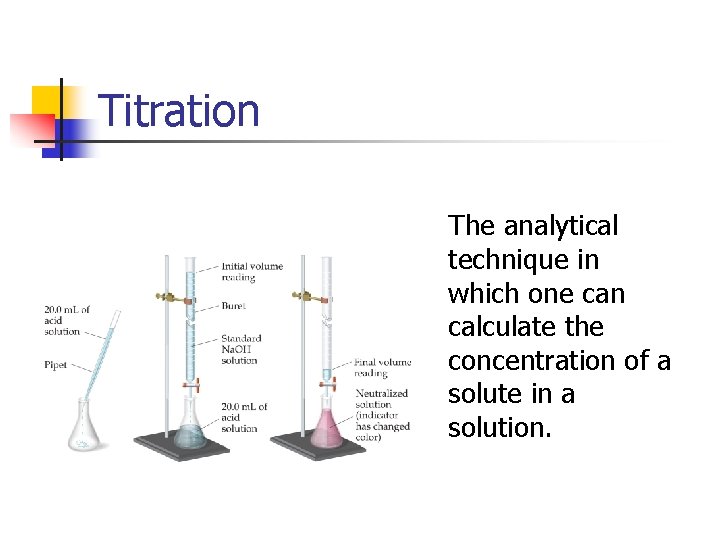
Titration The analytical technique in which one can calculate the concentration of a solute in a solution.

What volume of a 1. 420 M Na. OH solution is Required to titrate 25. 00 m. L of a 4. 50 M H 2 SO 4 solution? 4. 7