Querying Four Decades of Clinical Research Data at




















- Slides: 35

Querying Four Decades of Clinical Research Data at the National Institutes of Health James Cimino, MD, FACMI, FACP Laboratory for Informatics Development NIH Clinical Center and National Library of Medicine

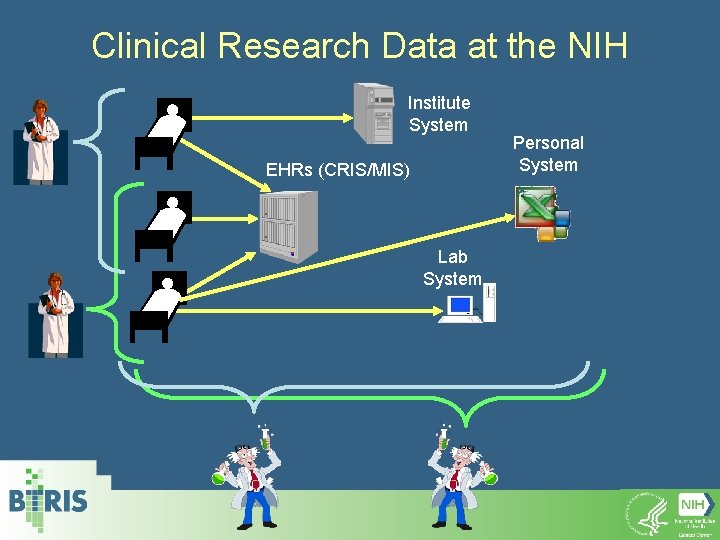
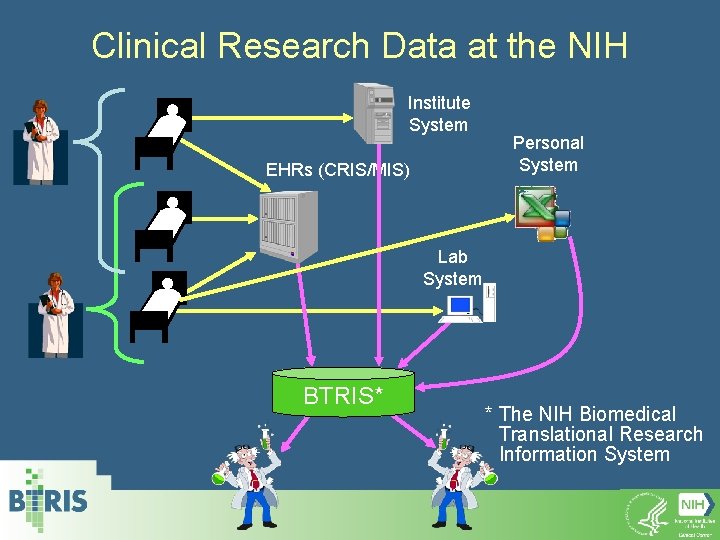
Clinical Research Data at the NIH Institute System EHRs (CRIS/MIS) Lab System Personal System

Clinical Research Data at the NIH Institute System EHRs (CRIS/MIS) Personal System Lab System BTRIS* * The NIH Biomedical Translational Research Information System

What is in BTRIS? • • CRIS – Alerts – Allergies – Anatomic Pathology – Blood Bank – Clinical Documents (unstructured) – Clinical Documents (structured) – Demographics – Diagnoses/Problems – Echocardiograms – Electrocardiograms – Lab Tests and Panels (Location) • – Medication Administration – Medication Orders – Microbiology (Links to Mass Spec) – PDF Documents – Radiology Reports – Radiology Images – Vital Signs MIS – Blood Bank – Demographics – Lab Tests and Panels – Medications – Microbiology – Radiology Reports – Vital Signs Other institutes – NIAID (CRIMSON: Labs, Meds, Problems) – NIAAA (Assessments) – NCI (Labmatrix, C 3 D, Biospecimens) – NICHD (CTDB: Forms) – NHGRI (Labmatrix, Exome Data)

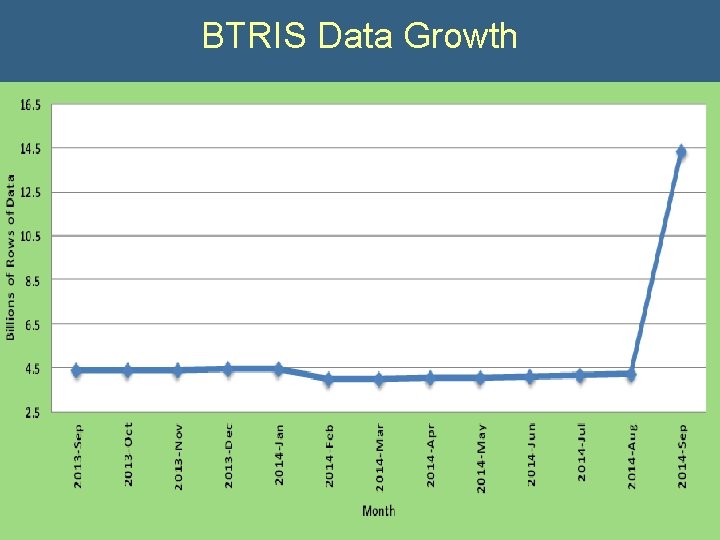
BTRIS Data Growth

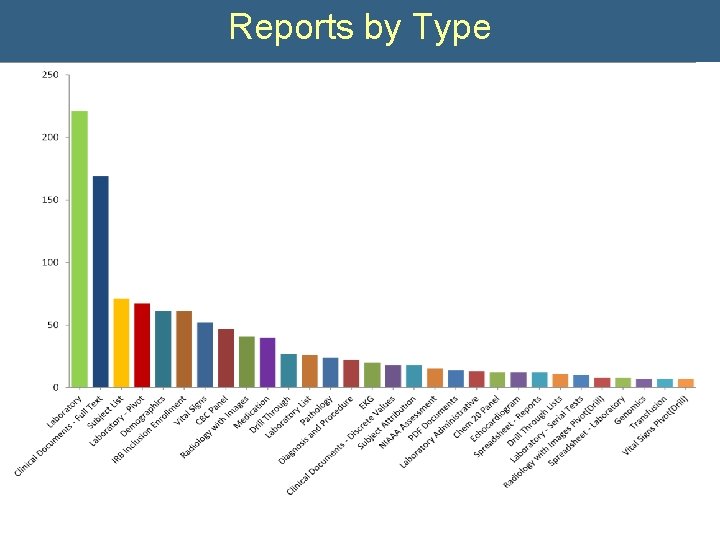
Reports by Type

BTRIS Functions • Identified data: – Attribute subjects to protocols – Query for protocol-related data – Submit summary results to Clinical. Trials. gov














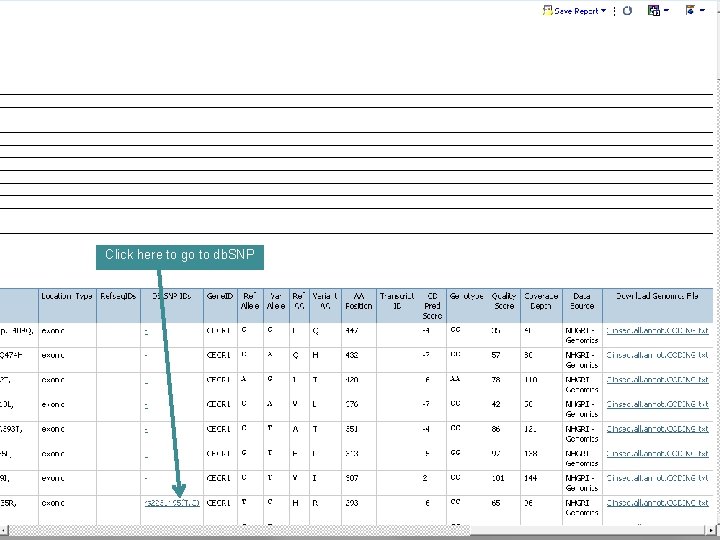
Click here to go to db. SNP


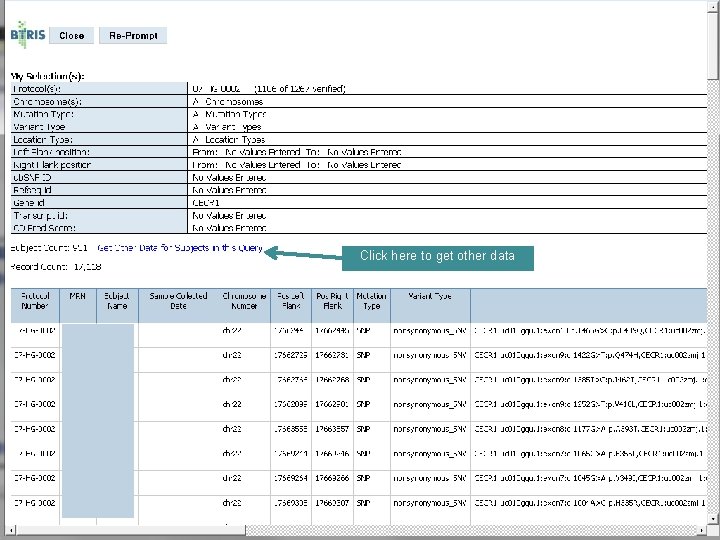
Click here to get other data


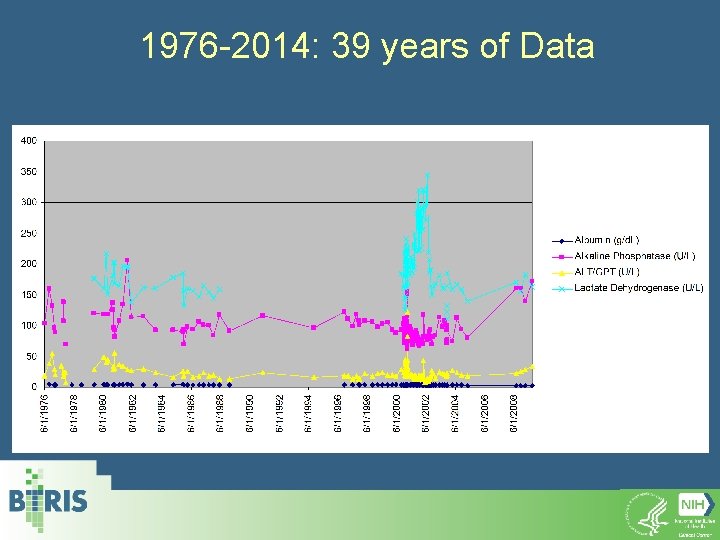
1976 -2014: 39 years of Data

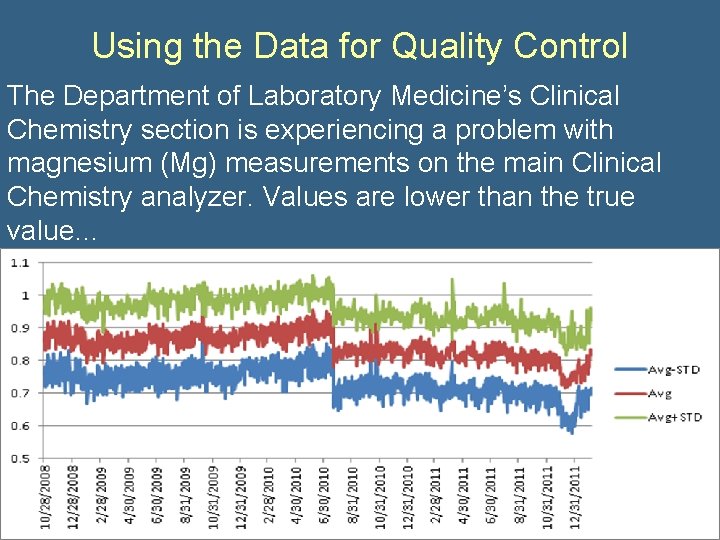
Using the Data for Quality Control The Department of Laboratory Medicine’s Clinical Chemistry section is experiencing a problem with magnesium (Mg) measurements on the main Clinical Chemistry analyzer. Values are lower than the true value…

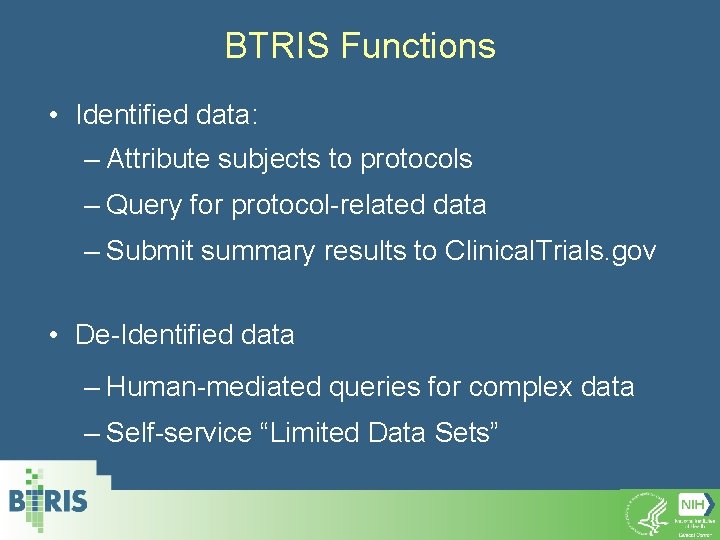
BTRIS Functions • Identified data: – Attribute subjects to protocols – Query for protocol-related data – Submit summary results to Clinical. Trials. gov • De-Identified data – Human-mediated queries for complex data – Self-service “Limited Data Sets”

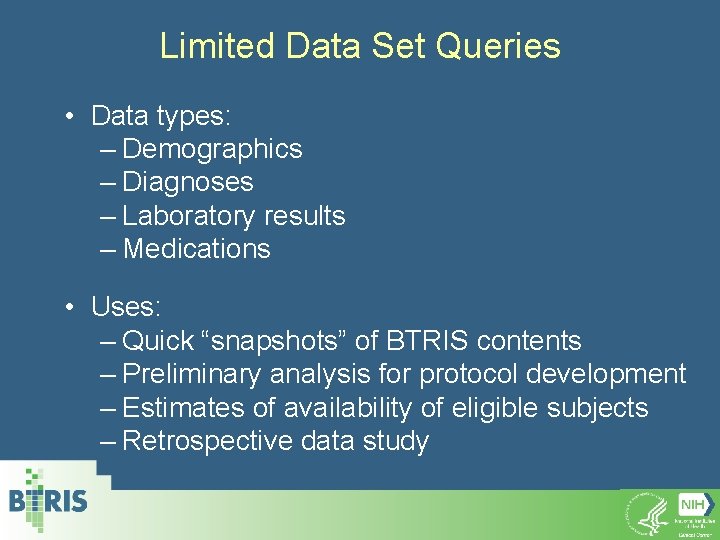
Limited Data Set Queries • Data types: – Demographics – Diagnoses – Laboratory results – Medications • Uses: – Quick “snapshots” of BTRIS contents – Preliminary analysis for protocol development – Estimates of availability of eligible subjects – Retrospective data study

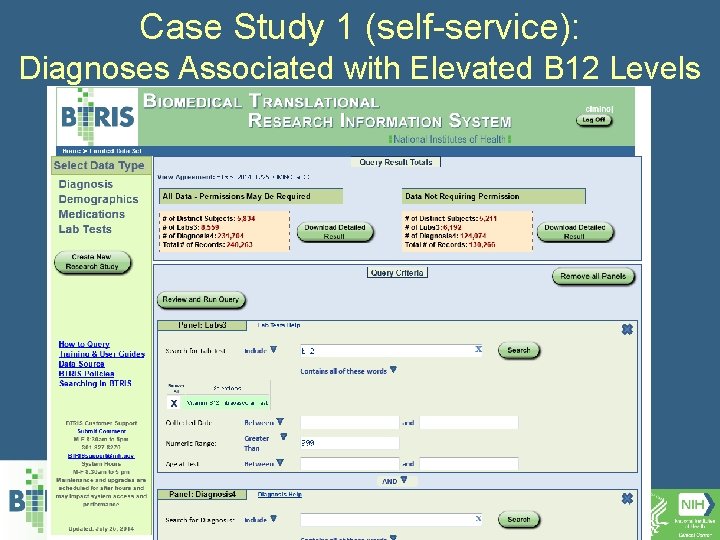
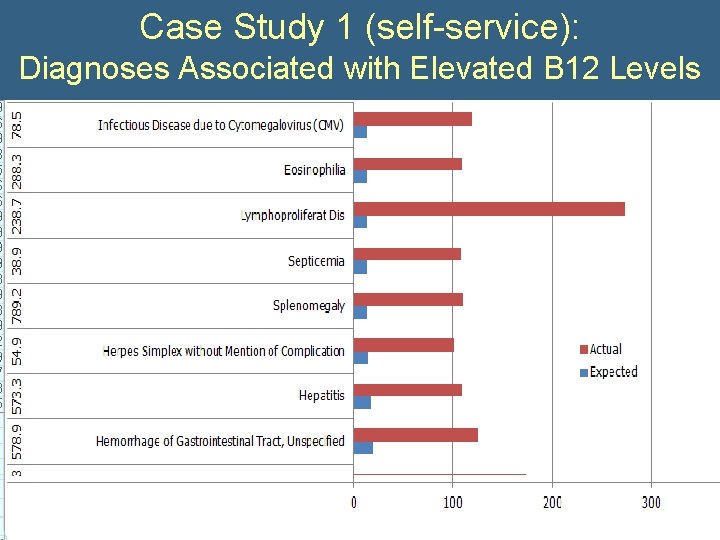
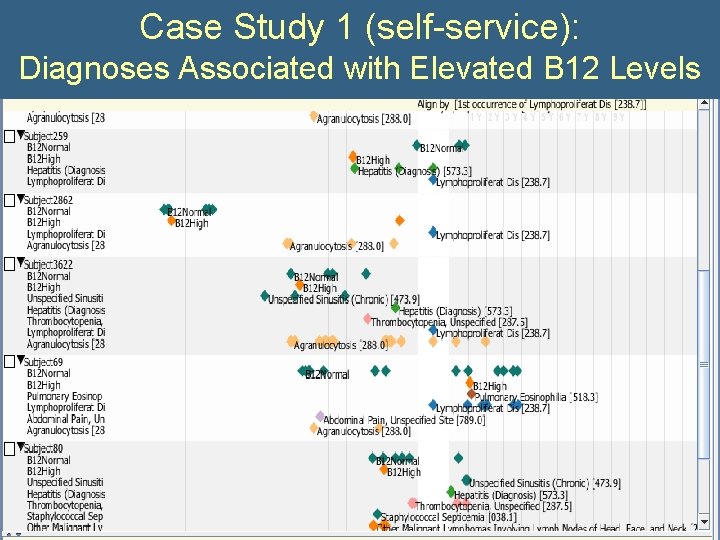
Case Study 1 (self-service): Diagnoses Associated with Elevated B 12 Levels • What is the significance of an incidental elevation of vitamin B 12? • Query: – All subjects with B 12>999 – All Diagnoses – All B 12 levels

Case Study 1 (self-service): Diagnoses Associated with Elevated B 12 Levels

Case Study 1 (self-service): Diagnoses Associated with Elevated B 12 Levels

Case Study 1 (self-service): Diagnoses Associated with Elevated B 12 Levels

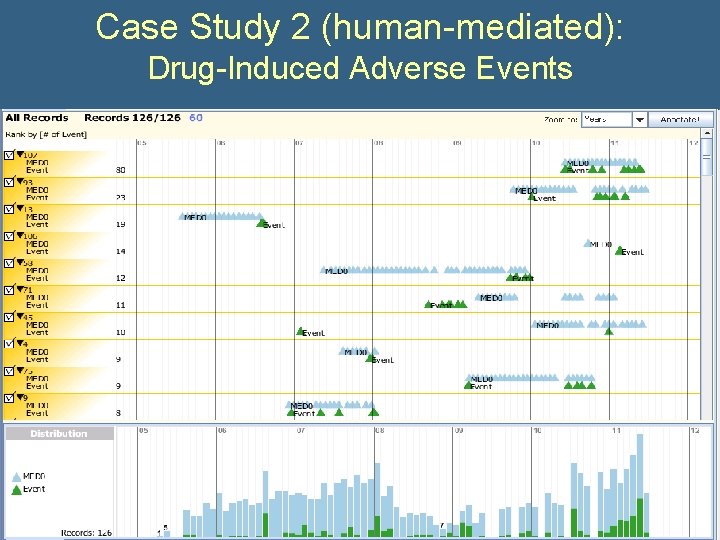
Case Study 2 (human-mediated): Drug-Induced Adverse Events • What is the correlation between a chemotherapeutic drug and adverse events over time? • Query: – All subjects with any dose of the drug – All systolic blood pressure < 90 mm. Hg

Case Study 2 (human-mediated): Drug-Induced Adverse Events

What Does Really BTRIS Add? • Centralization and curation of data • Centralization and curation of terminologies • Self-service tools • Clarification and operationalization of policies
 Access module 2: querying a database
Access module 2: querying a database Access module 2 querying a database
Access module 2 querying a database Decades project examples
Decades project examples Last decades art
Last decades art One step forward two decades back
One step forward two decades back Stages of clinical supervision
Stages of clinical supervision What is socra
What is socra Dr lemech
Dr lemech Research design in clinical psychology
Research design in clinical psychology Pi clinical research consultancy
Pi clinical research consultancy Good documentation practices in clinical research
Good documentation practices in clinical research Clinical research statistician
Clinical research statistician Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Kavi institute of clinical research
Kavi institute of clinical research Aro clinical research
Aro clinical research Translating research findings to clinical nursing practice
Translating research findings to clinical nursing practice Jasper clinical research
Jasper clinical research Asbmt clinical research training course
Asbmt clinical research training course Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Clinical research support services
Clinical research support services Clinical research definition
Clinical research definition Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Questra clinical research
Questra clinical research Mrc career development award
Mrc career development award Foundations of clinical research applications to practice
Foundations of clinical research applications to practice Iwr clinical research
Iwr clinical research Mghs wheelers
Mghs wheelers Society of clinical data management
Society of clinical data management Clinical data repository
Clinical data repository Sae reconciliation steps
Sae reconciliation steps Four sides and four corners
Four sides and four corners Skin assessment charting
Skin assessment charting Dimensions of research
Dimensions of research