Society for Clinical Data Management Draft Definition of









- Slides: 12

Society for Clinical Data Management Draft Definition of Clinical Research Data Management (CRDM) Meredith Nahm, Ph. D Duke Center for Health Informatics Thursday, March 21, 2013 AMIA CRI Working Group Meeting

Society for Clinical Data Management (SCDM) • non-profit professional association • Started in 1994 • Mission: to support Clinical Data Management professionals • Vision: to be the world’s leading advocate for the discipline of clinical data management. • Membership 2400, 80% US, 20% international

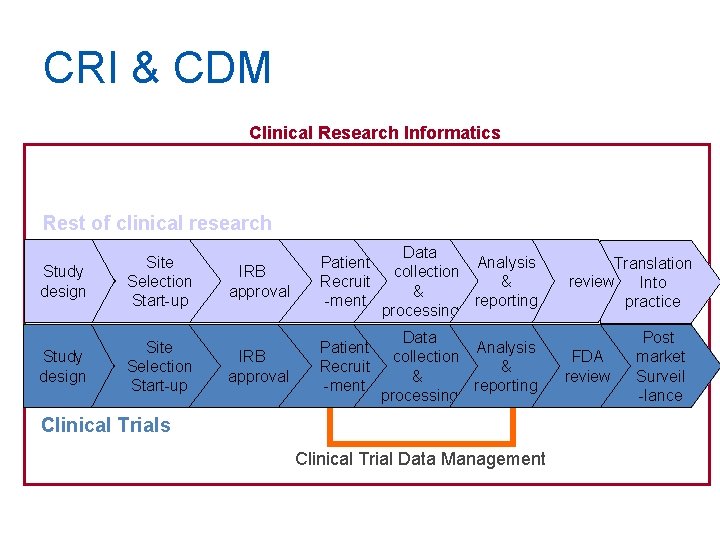
CRI & CDM Clinical Research Informatics Rest of clinical research Study design Site Selection Start-up IRB approval Patient Recruit -ment Data Analysis collection & & reporting processing Clinical Trials Clinical Trial Data Management Translation review Into practice FDA review Post market Surveil -lance

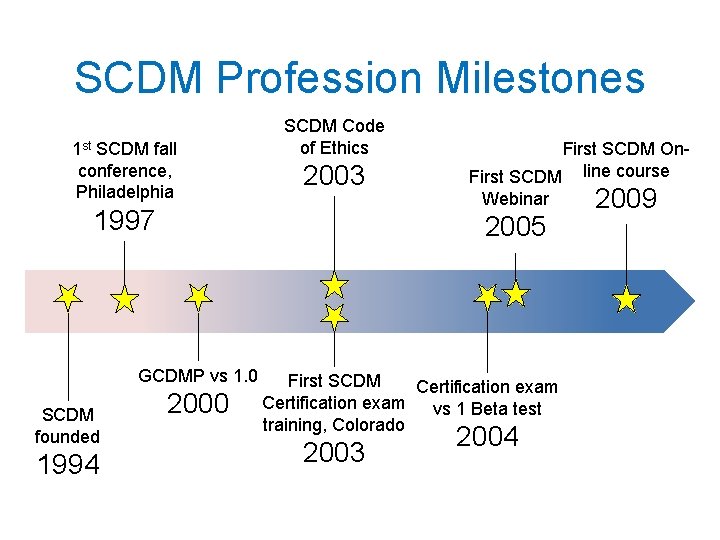
SCDM Profession Milestones 1 st SCDM fall conference, Philadelphia SCDM Code of Ethics 2003 1997 1994 Webinar 2005 GCDMP vs 1. 0 SCDM founded First SCDM On. First SCDM line course 2000 First SCDM Certification exam vs 1 Beta test training, Colorado 2003 2004 2009

From US to International Member of INCDMA First international conference: Toronto 2004 Transferred mgt. contract to international association mgt. company 2011 Second international conference: Mumbai 2013

What does SCDM do ? • monthly e-newsletter, Data Connections • quarterly digital peer-reviewed journal (not indexed), Data Basics • Good Clinical Data Management Practices (GCDMP) maintained since version 1. 0 in 2000 – English and Japanese – Referenced in FDA draft guidance • Certification (CCDM): 596 current CCDMs • Professional development courses

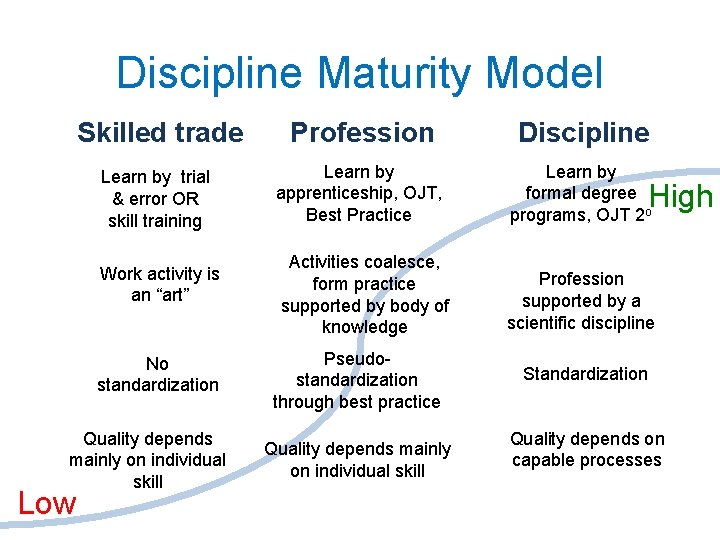
Discipline Maturity Model Skilled trade Profession Discipline Learn by trial & error OR skill training Learn by apprenticeship, OJT, Best Practice Learn by formal degree programs, OJT 2 o Activities coalesce, form practice supported by body of knowledge Profession supported by a scientific discipline Work activity is an “art” No standardization Quality depends mainly on individual skill Low Pseudostandardization through best practice Quality depends mainly on individual skill High Standardization Quality depends on capable processes

We Are What We Know And, what we know grows and evolves year after year as basic and applied informatics research generates new knowledge. Fall meeting Sept 22 -25, presented a draft definition of the profession for comment.

Updated Draft Definition Purpose of the definition: explicit articulation of the goals, value and products of the profession Three new things: 1) recognition of Informatics as underlying science 2) narrowing of the name to research, i. e. , CRDM rather than CDM 3) in response to broadening roles in industry acknowledgement of and intention to support professionals across the full spectrum of the NIH definition of clinical research … not just RCTs

Comments • Vetted at Fall 2012 SCDM annual meeting • Received 42 comments 19 Persuasive Supportive 17 Not persuasive 5

Current Definition Clinical Research Data Management is the expert application of informatics theories and methods to the definition, collection and processing of data for clinical studies and the design of associated work and data flow. Clinical Research Data Management assures the collection, integration and availability of data at appropriate quality and cost. Clinical Research Data Management supports the conduct, management and analysis of studies across the spectrum of clinical research, and ultimately public health and confidence in therapeutics and therapeutic interventions.

Next Steps • Sharing in the AMIA CRI-wg • Sharing again with SCDM membership Send comments to: meredith. nahm@duke. edu