QOD 2016 2017 DSQ 91 What is chemistry





















- Slides: 29

QOD 2016 -2017

DSQ • 9/1 - What is chemistry? • 9/2 - Name 3 lab safety rules. • 9/6 - Name 2 pieces of lab equipment used in the lab and describe their function? • 9/7 - What is the difference between an observation and an inference? • 9/8 - How are accuracy and precision the same? Different?

DSQ • 9/9 - How do we determine an object’s shape if we can’t see it or touch it? • 9/12 – After completing the Mystery box lab, how reliable do you believe the method of indirect measurement and the inferring of explanations from experiments with observable results is? • 9/13 -Which of the following scientists is NOT responsible for contributing to the discovery of atomic structure? – Thomson – Einstein – Dalton – Rutherford

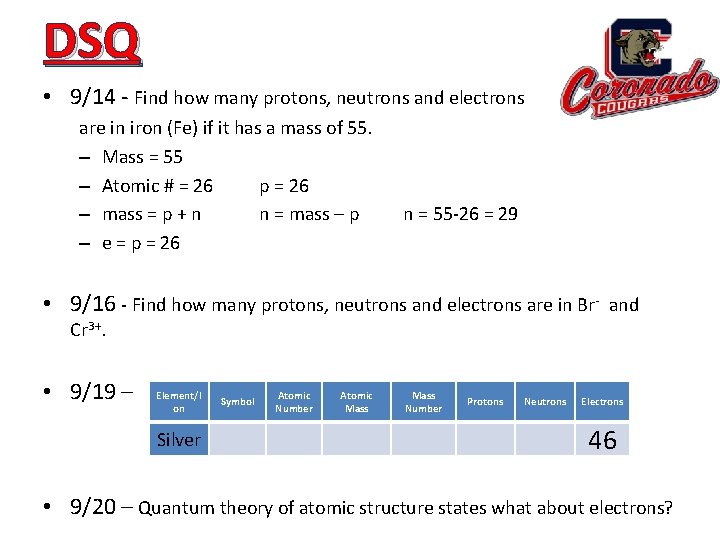
DSQ • 9/14 - Find how many protons, neutrons and electrons are in iron (Fe) if it has a mass of 55. – Mass = 55 – Atomic # = 26 p = 26 – mass = p + n n = mass – p – e = p = 26 n = 55 -26 = 29 • 9/16 - Find how many protons, neutrons and electrons are in Br- and Cr 3+. • 9/19 – Element/I on Silver Symbol Atomic Number Atomic Mass Number Protons Neutrons Electrons 46 • 9/20 – Quantum theory of atomic structure states what about electrons?

DSQ • 9/21 - What is the total number of electrons that can be held in the third principal energy level? • 9/22 – Write the electron configuration for Radium (Ra). • 9/23 – Write (both the long and short way) and draw the electron configuration for Cr. • 9/26 – Write the electron configuration for Ga+3 • 9/27 – Draw the electron configuration for Hg 2+ • 9/28 – Draw the electron configuration for O-3 • 9/29 – Write the shorthand electron configuration for Ra-3 • 9/30 – Atomic Structure Test

DSQ • 10/4 - Mendeleev published a table of the elements organized by increasing what? Which scientist arranged elements on the periodic table according to atomic number? • 10/5 - More than two-thirds of the elements on the periodic table are what? If solid, non metals tend to be what? • 10/6 – The elements along the stair step line are called what? What are their characteristics? • 10/7 - What element is found in Group 14 and in Period 2? What is the number of valence electrons in an atom with an electron configuration of 1 s 22 p 63 s 23 p 3? • 10/10 - What is the element with the electron configuration of: [Xe]6 s 24 f 145 d 106 p 3? How many valence electrons?

DSQ • 10/11 - When moving across a period in the periodic table, why does the atomic radii become smaller? – Because the energy level of across the period is the same, the positive centers of atoms pulls the e- cloud closer to the nucleus, making the atom smaller. • 10/12 – No Question • 10/13 - What does electronegativity of an element measure? • 10/14 – What is the relationship between atomic radius and ionization energy?

DSQ • 10/17 – The unit used to indicate the volume of a • • regular object? The unit used to indicate the volume of an irregular object? 10/18 - 28. 5 g of iron shot is added to a graduated cylinder containing 45. 50 m. L of water. The water level rises to the 49. 10 m. L mark, from this information, calculate the density of iron. 10/19 - PSAT 10/20 - An unknown substance from planet X has a density of 10 g/ml. It occupies a volume of 80 ml. What is the mass of this unknown substance? 10/21 - Test

DSQ • 10/25 – What characteristic defines the different atoms? What are the same atoms with different number of neutrons called? • 10/26 – What is radioactivity? • 10/27 – What are the 2 types of nuclear radiation? • 10/31 – Thorium-238 undergoes alpha decay. Write the equation. • 11/1 – Write the equation for the beta decay of carbon-14 • 11/2 – Bismuth-210 undergoes two types of radioactive decay: first beta then alpha. Write the nuclear equation for each radioactive decay. (Hint – the daughter isotope of the first decay becomes the parent isotope of the second decay)

DSQ • 11/3 – What is half-life? • 11/4 – How many minutes will it take for a 30. 0 g sample of Se-83 to decay to 1. 875 g if the half life is 20. 5 minutes? • 11/7 – Actinium-226 has a half life of 29 hours. After a period of 87 hours, 2. 5 g of the isotope remains. How large was the original sample? • 11/8 – no school • 11/9 – If 12. 5% of isotope Q remains after 36 minutes, what is it’s half-life? • 11/10 – Nuclear Chemistry Test

DSQ • 11/15 – Name and explain the three types of chemical bonds? • 11/16 - Draw the Lewis Dot Structure for the following: Sr + P • 11/17 - Draw the Lewis Dot Structure for the following: 1. Na + S 2. H 2 O • 11/18 - Draw the Lewis Dot Structure for CCl 4 • 11/28 – Identify the type of bonding and draw the Lewis Dot Structure for the following: 1. K + O 2. CO 2

DSQ • 11/29 – Draw the Lewis Dot Structure for the following: Cl. O 4 • 11/30 – Draw the Lewis Dot Structure for the following: PO 33 • 12/1 - Draw the molecular geometry for the following: SF 6 • 12/2 - Draw and name the molecular geometry for Br. F 5 • 12/5 - Are the following molecules polar or nonpolar? OF 2 CCl 4 • 12/6 - Bonding Test

DSQ • 12/8 – What does a phase diagram show? • 12/9 – What physical state is this substance in at 5. 1 atm and 310 C? • 12/13 - Determine the IMF for IF 5 • 12/14 – Name the IMF and rank in order of strength: NH 3, I 2, CO 2 • 12/15 – What is the relationship between IMF and melting point and boiling point?

DSQ 1/4 – How many atoms are in 2 C 7 H 8 N 4 O 2 (chocolate) ? 1/6 – Write the formula for ammonium phosphate? 1/9 – What is the stock name of Fe 2(SO 3)3 ? 1/10 – Write formula for vandium(III) thiocyanate. 1/11 – Write the formula for lead(II) hydroxide. 1/18 – 1/20 Semester 1 mid term exams 1/23 - Write the name of P 2 Cl 4 and the formula for calcium carbonate (marble). • 1/24 - Write the name of Fe(NO 3)3 and N 2 O 2. • •

DSQ 1/25 – What is the difference between SO 3 -2 and SO 3 ? 1/26 – Write the name of H 2 Cr. O 4. 1/27 – Write the formula for arsenic acid. 1/30 - Write the name of HBr. O 3. Write the formula for hydrobromic acid. • 1/31 – Nomenclature test • 2/2 - Balance the following equation: ____ Rb. NO 3 + ____ Be. F 2 ____ Be(NO 3)2 + ____ Rb. F • 2/3 – Balance the following equation: ____ Al. Br 3 + ____ K 2 SO 4 ____ KBr + ____ Al 2(SO 4)3 • •

DSQ • 2/6 – Balance the following equation: ____ C 3 H 18 + ____O 2 ____ CO 2 + ____ H 2 O • 2/7 – Balance the following equation: ____ C 0 + ____Fe 2 O 3 ____ CO 2 + ____ Fe • 2/8 – Balance the following equation: Sodium Bicarbonate yields Sodium Carbonate + Carbon Dioxide + Water • 2/9 - Aqueous iron (III) chloride and sodium carbonate solution yields aqueous sodium chloride and a precipitate of iron (III) carbonate.

DSQ • 2/13 – Write, balance and determine the reaction type: Potassium bromide solid when mixed with fluorine produces potassium fluoride solid and bromine. • 2/14 – Write, balance and determine the reaction type: nickel(II) chlorate solution -> nickel(II) chloride solution + oxygen gas • 2/15 – Write, balance and determine the reaction type: Sodium metal is placed in phosphoric acid and produces dissolved sodium phosphate and hydrogen gas. • 2/16 – What is solubility?

DSQ • 2/17 – Write the ionic equation for H 2 Se. O 4 • 2/21 – Write the net ionic formula for: Mg(NO 3)2 + Na 2 CO 3 Mg. CO 3 + Na. NO 3 • 2/22 – Chemical Reaction Test • 2/24 – Convert 0. 004 k. L to _______m. L • 2/27 – First write this equation in scientific notation then solve: 245600 x 0. 0065 = • 3/1– The drive to school take Josh 15 minutes and is 5. 78 miles. How many cm is this?

DSQ • 3/2– According to the specifications in the owner’s manual for Sandra’s compact car, the combined "capacity weight" of the occupants and luggage is 385 kilograms. Calculate the "capacity weight" of her car in ounces. • 3/3 – Solve: (2. 32 × 10− 6)/(4 × 10− 5) • 3/8 - Calculate the number of ions in 6. 5 moles of Au 2+? • 3/9– Calculate the number of moles from 3. 25 x 1021 Au 2+ ions? • 3/10– Calculate the number of moles from 9. 03 x 1024 formula units of Sodium Nitrate?

DSQ • 3/13 – Calculate the formula mass for Iron(III) sulfate. • 3/14 – How many grams is 3. 2 moles of copper(II) carbonate. • 3/15 - How many moles are in 50 grams of sodium bicarbonate (baking soda)? • 3/16 – How many moles are in a 255 gram sample of barium phosphate? How many formula units is this? • 3/17 – When converting between moles <-> particles, what conversion factor is used? When converting between moles <-> mass (g), what conversion factor is used?
DSQ • 3/20 – How many grams are in 1. 78 x 1023 molecules of H 2 O? • 3/21 - How many atoms are in 59 g of P 2 O 5? • 3/22 – Mole Quiz • 3/23 – How do you make a S’more? • 3/24 – Using the equation for S’mores: 2 S + 1 Mm +4 Or -> 1 S 2 Mm. Or 4 What is the ratio between chocolate and S’mores? How many S’mores can be made with 96 pieces of chocolate?

DSQ • 3/27 – Write a balanced equation include ratio and rxn type: copper(II) chloride + sodium sulfide -> sodium chloride + copper(II) sulfide • 3/28 - Sodium chlorate decomposes to form sodium chloride and oxygen gas. How many grams of oxygen are produced using 12. 00 grams of sodium chlorate? • 3/29 - How many grams of zinc metal are needed to replace 5. 00 grams of silver nitrate solution which forms zinc nitrate solution and silver metal. • 3/30 - How many grams of beryllium oxide solid can be produced when 50. 0 grams of beryllium metal is combined with excess atmospheric oxygen in a synthesis reaction

DSQ • 4/3 – Given the S’more formula: S 2 Mm. Or 2. How many S’mores could you make with 9 graham cracker squares (S), 5 marshmallows (Mm) and 12 pieces of chocolate (Or)? Which ingredient limits the number of S’mores you can make? • 4/4 - A 2. 00 g sample of ammonia (NH 3)is mixed with 4. 00 g of oxygen to produce nitrogen monoxide and water. Which is the limiting reactant? 4 NH 3(g) + 5 O 2(g) -> 4 NO(g) + 6 H 2 O(g) • 4/5 – Write a balanced equation for the double replacement reaction of lead(II) nitrate and potassium iodide. • 4/6 - Oxygen gas and iron metal react to form Iron(II) oxide ash. In an experiment, 75. 0 grams of oxygen combines with 100. 0 grams of iron. Determine which reactant is limiting and calculate the grams of product produced. Calculate the percent yield if 125. 7 g Iron(II) oxide were produced in the lab.

DSQ • 4/7 – Stoichiometry Test • 4/18 – Write the name of H 2 Cr 2 O 7 ; Write the formula for Boric Acid • 4/19 - According to Bronsted-Lowry theory, what is an acid and what is a base? • 4/20 – What is the molarity of 245. 0 g of H 2 SO 4 dissolved in 1. 00 L of solution? • 4/21 – What is the weight (in grams) of HCl would be needed to make 500 m. L of a 1. 50 M solution? • 4/24 – Science EOC testing • 4/25 - Science EOC testing • 4/26 – What is the concentration of base needed if 625 ml of Li. OH is used to neutralize 350 ml of 2. 5 M HF? • 4/27 – What is the p. H of a 6. 50 x 10 -3 M KOH solution?

DSQ • 4/28 – What is the p. H of a. 25 M Sr(OH)2 solution? • 5/1 – Calculate the base concentration give 0. 350 M HF (3. 5% dissociation). • 5/2 – EOC testing • 5/3 - EOC testing • 5/4 – EOC testing • 5/5 - EOC testing

DSQ • 5/8 – The temperature of the human body is 37 o C. What is it in Kelvin (K)? • 5/10 – If 15 L of air is added to a balloon at sea level (1 atm) and the balloon is taken to Denver where the air pressure is 0. 85 atm. What will be the new volume of the balloon? • 5/11 – On hot days you may have noticed unopened potato chip bags seem to “inflate”. If I have a 250 m. L bag at a temperature of 19 o. C and I leave it in my car which has a temperature of 60 o. C, what will the new volume of the bag be?

DSQ • 5/12 – The temperature inside my refrigerator is about 4 o C. If I place a balloon in my refrigerator that initially has a temperature of 22 o C and a volume of 0. 5 L, what will be the volume of the balloon when it is fully cooled? • 5/15 - A sample of oxygen gas has a temperature of 14. 5 o. C, a volume of 500. m. L, and a pressure of 850. 0 torr. What would be the new volume of the oxygen at STP? • 5/16 - I have a gas at an initial pressure of 1. 1 atm and -10. 0 o. C, what will the temperature be if I increase the pressure to 3. 4 atm? • 5/17 – A 28 L sample of gas at 45 o. C and unknown pressure has its volume increased to 34 L and its temperature decreased to 35 o. C. If the pressure after the change is 2. 0 atm, what was the original pressure of the gas?

DSQ • 5/18 – EOC testing • 5/19 – EOC testing • 5/22 - A sample of 4. 25 moles of hydrogen at 20. 0 C occupies a volume of 2500 m. L. Under what pressure is this sample? • 5/23 - What will have to happen to the temperature of a sample of methane if 1000. m. L at 98. 6 k. Pa and 25. 0 C occupies a new volume of 900. m. L with no change to the pressure? • 5/24 - A gas is heated to 80. 0 C and a pressure of 2. 0 atm. If the container expands to hold a volume of 800. m. L, what was the volume of the gas at a temperature of 50. 0 C and 930 mm. Hg pressure?

DSQ • 5/25 – Blast furnaces give off many unpleasant and unhealthy gases. If the total air pressure is 0. 990 atm, the partial pressure of carbon dioxide is 0. 050 atm, and the partial pressure of hydrogen sulfide is 0. 020 atm, what is the partial pressure of the remaining air?
 Visualiseur dsq
Visualiseur dsq Qod drug
Qod drug January 2018 regents chemistry
January 2018 regents chemistry 2017 ap chem exam
2017 ap chem exam Inorganic vs organic chemistry
Inorganic vs organic chemistry Ib organic chemistry
Ib organic chemistry Microsoft threat modeling tool
Microsoft threat modeling tool Single 2016
Single 2016 Data center switching gartner
Data center switching gartner Kotler p. armstrong g. principles of marketing
Kotler p. armstrong g. principles of marketing 2016 lys tarih
2016 lys tarih Afs 2016:1
Afs 2016:1 Microsoft office 2016 in practice
Microsoft office 2016 in practice 28 februari 2016
28 februari 2016 Fgv 2016
Fgv 2016 Xxxxxxx the
Xxxxxxx the Astro quiz 2018 questions and answers
Astro quiz 2018 questions and answers Permen lhk p.68 tahun 2016
Permen lhk p.68 tahun 2016 2016 extenso
2016 extenso Financial management operations manual (fmom) pdf
Financial management operations manual (fmom) pdf Master data services import type
Master data services import type Descopera intrusul 876
Descopera intrusul 876 Where is the love 2016 lyrics
Where is the love 2016 lyrics Testout server pro 2016
Testout server pro 2016 Pat acer oars
Pat acer oars Butler 2016
Butler 2016 Zpg biologie
Zpg biologie Tni standard
Tni standard 2016 pearson education inc
2016 pearson education inc 2016 ssef abstract form
2016 ssef abstract form